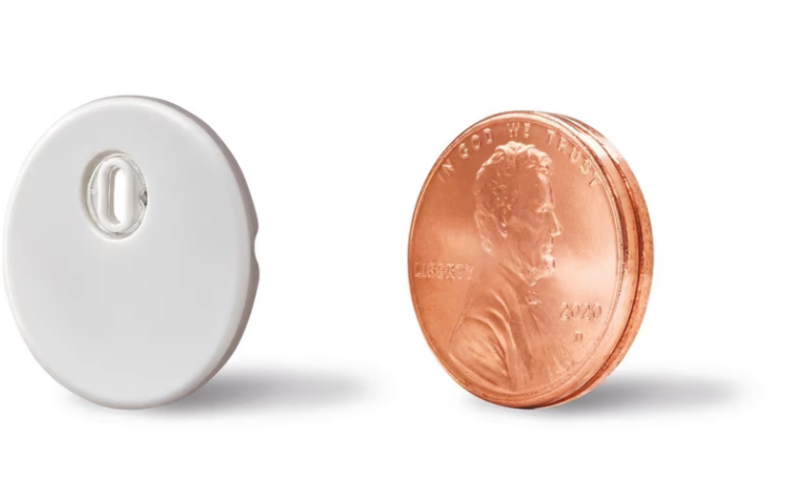Nearly two years after going live in Europe, the latest version of Abbott’s FreeStyle Libre continuous glucose monitor is ready to make its stateside debut.
The FDA cleared the FreeStyle Libre 3 system, Abbott said Tuesday, and the company will begin rolling out the glucose sensor to participating pharmacies later this year.
The device is intended for people aged four years and older with either Type 1 or Type 2 diabetes. It uses a sensor that sticks to the back of the arm for up to two weeks at a time and automatically sends glucose readings to a Bluetooth-connected smartphone every minute.
The FreeStyle Libre 3 system isn’t yet eligible for Medicare reimbursement, but Abbott said it’s aiming to price the monitor at the same rate as previous iterations of the system so it can be widely accessible to more people with diabetes.
“We continue to disrupt the notion that CGMs have to sacrifice quality or accuracy for affordability,” said Jared Watkin, senior vice president of Abbott’s diabetes care business. “Access to breakthrough diabetes technologies should not be out of reach for the people who can benefit most from them.”
The newly cleared sensor is Abbott’s smallest yet, measuring in at the width of just two stacked pennies. The company also made the sensor easier to apply than previous versions: The FreeStyle Libre 3 can be placed on the back of the arm using just a one-piece applicator.
Sensor-based CGM systems like Abbott’s are designed to provide blood sugar readings without requiring users to perform regular fingersticks. According to Abbott, the FreeStyle Libre 3 boasts an overall mean absolute relative difference, or MARD—a measure of the rate of errors in a device’s readings—of 7.9%, making it the first to claim a sub-8% error rate.
The sensor’s Bluetooth signal stretches up to 33 feet, which Abbott claims is 50% further than the ranges of other CGM sensors on the market, including the Dexcom G6 and Medtronic Guardian Connect devices.
The sensor sends minute-by-minute updates to the FreeStyle Libre app on a connected smartphone, which users can program to issue alerts if their glucose readings leave a specified range. Abbott also offers the LibreView and LibreLinkUp apps to give healthcare providers and caregivers real-time access to the readings.
The system’s U.S. clearance follows its CE mark approval in Europe, which arrived in September 2020.
The stateside clearance also comes shortly after Abbott unveiled data showing that the entire FreeStyle Libre family of glucose monitors are linked to strong glucose management. A study published in September showed that patients who use the system’s smartphone app to track their blood sugar readings spend about 5% more time in their ideal glucose range than those who manually track readings.
Another analysis published in April suggested that not only is the FreeStyle Libre system linked to significant reductions in glucose levels, it does so at similar levels in both Type 1 and Type 2 diabetes patients.
The FreeStyle Libre portfolio has also had a strong showing financially as of late. Altogether, the portfolio brought in $1 billion in the first quarter of this year, representing more than 8% of Abbott’s entire sales take for the period and a 20% increase compared to last year.