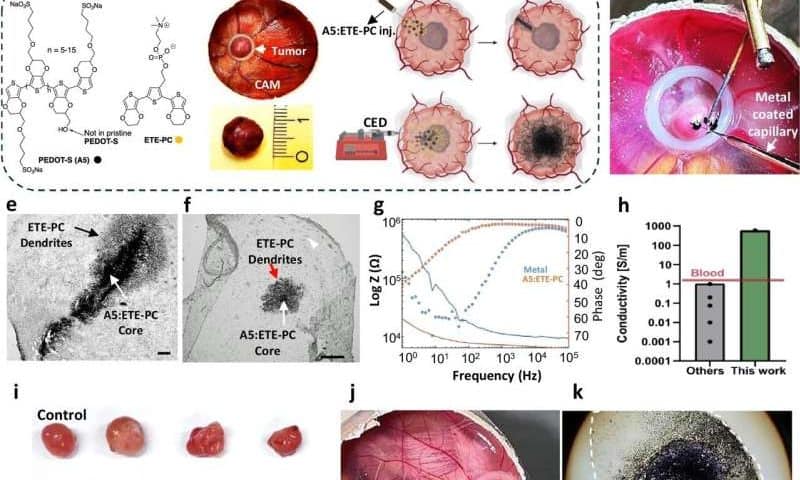
Electrotherapy using injectable nanoparticles delivered directly into the tumor could pave the way for new treatment options for glioblastoma, according to a new study from Lund University in Sweden.
Glioblastoma is the most common and most aggressive form of brain tumor among adults. Even with intensive treatment, the average survival period is 15 months. The tumor has a high genetic variation with multiple mutations, which often makes it resistant to radiation therapy, chemotherapy and many targeted drugs. The prognosis for glioblastoma has not improved over the past few decades despite extensive research.
How electrotherapy targets tumors
Electrotherapy offers another strategy to combat solid tumors. Using short, strong electric pulses (irreversible electroporation), non-reversible pores are created in the cancer cells leading to their death. The body’s immune system is simultaneously stimulated.
The problem is that surgery is required to place the stiff metal electrodes that are necessary for the treatment. In sensitive tissue, in the brain for example, this often entails a very difficult procedure, which has led to strict criteria regarding which patients can be treated.
Johan Bengzon is a researcher in glioblastoma and adjunct professor at Lund University, and consultant in neurosurgery at the Skåne University Hospital. He regularly treats patients with glioblastoma and is frustrated by the limited treatment options.
“The short distance between the hospital and the University in Lund facilitates cooperation and that’s why I contacted research colleagues to find out if injectable electrodes could be an alternative solution in electrotherapy,” says Johan Bengzon.
Injectable nanoparticles as a new approach
The research team, with Amit Singh Yadav, Martin Hjort, and Roger Olsson at the helm, had previously used nanoparticles to form injectable and electrically conductive hydrogels to control brain signaling and heart contractions. It is a minimally invasive method in which the particles are injected using a thin syringe directly into the body.
The particles break down after the treatment and thus do not need to be surgically removed. Perhaps the same technology could be used to destroy tumor cells in glioblastoma.
“After surgical treatment, unfortunately the glioblastoma tumor often returns on the outer edge of the area operated on. By drop casting the nanoparticles into the tumor cavity after an operation, we could electrify the edges while the immune system is also activated. In animal models, the procedure, due to this irreversible electroporation, led to tumors being wiped out within three days,” says Olsson, professor of chemical biology and drug development at Lund University, who led the study.
Early results and future prospects
The prospects are good and the researchers are very hopeful for the future, even though there is a long way to go before it becomes a clinical reality. The challenge is now to test the method on larger tumors.
“We have seen that the electrode is well received in the brain. We have not noted any problems relating to side effects and after 12 weeks the electrode disappeared by itself as it’s biodegradable. The technology combines direct tumor destruction with activation of the immune system and can be an important step towards more effective treatment of glioblastoma,” concludes Singh Yadav, researcher at Lund University and first author of the study.