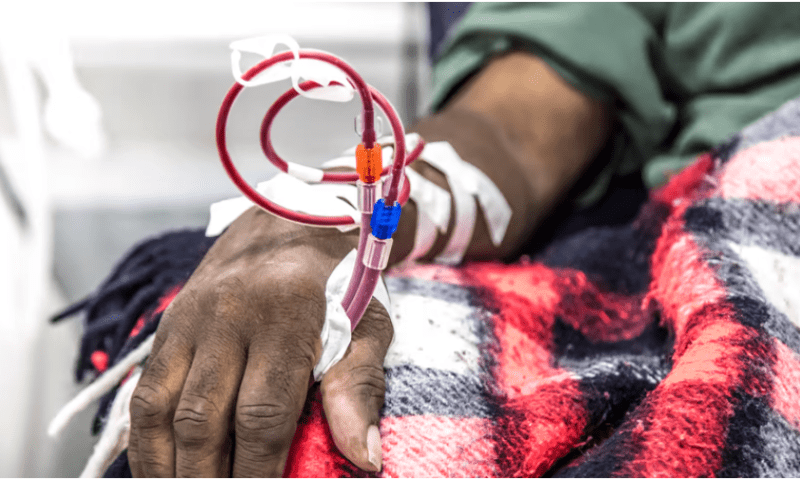
Medtronic has asked healthcare providers to immediately stop using more than 1 million catheters distributed across the U.S. and around the world after discovering a potential safety risk within the devices.
The alert marks the latest Class I recall for a company that has racked up half a dozen such safety events since the start of the year. The FDA dished out its most serious classification to the catheter recall last week, about a month after Medtronic initiated it in early June.
The recall covers a total of 1,019,414 hemodialysis catheters manufactured by Medtronic subsidiary Covidien, according to the FDA’s recall database. It spans several models of nine separate catheters marketed under the devicemaker’s Palindrome and Mahurkar product names. More than half of the affected devices are labeled as the Palindrome Chronic Catheter.
Each of the prescription-only, single-use devices was previously cleared by the FDA for use in acute and chronic hemodialysis, apheresis and blood infusion procedures. In a dialysis procedure, for example, the catheter is placed under the skin to draw out blood through one channel, or lumen, and return the cleaned blood back to the body through another.
Medtronic kicked off the recall last month after discovering a “potential leaking condition” within the catheters, according to a letter sent to affected healthcare providers at the time.
The issue stems from the presence of an empty space between the devices’ two lumens in the hub of the catheter where they meet. That manufacturing flaw could lead to “cross-communication of the blood circuit,” Medtronic said, where flushing one tube may mistakenly send liquid back through the other tube.
If that happens while the catheter is being used on a patient, it could potentially cause embolism, thrombosis, hemolysis, infection or simply inadequate treatment. Replacing the faulty catheters carries its own risks, including infection, a delay in treatment and additional radiation exposure during the placement process.
As of the recall’s June 8 start date, the medtech giant said it had received only one complaint regarding the catheter issue, with no reports of patient injuries or deaths linked to the flaw.
Healthcare providers are asked to compare their stores of unused Covidien catheters to the model and lot numbers of those included in the recall, and any that match should be immediately quarantined and returned to Medtronic.
Recalled catheters that are currently in use may not need to be removed or replaced. Instead, physicians should continually check to make sure the devices are working as needed. If any issues do arise, they should be immediately reported to Medtronic and the FDA, and the patient’s medical team can weigh the potential risks and benefits of replacing the catheter.