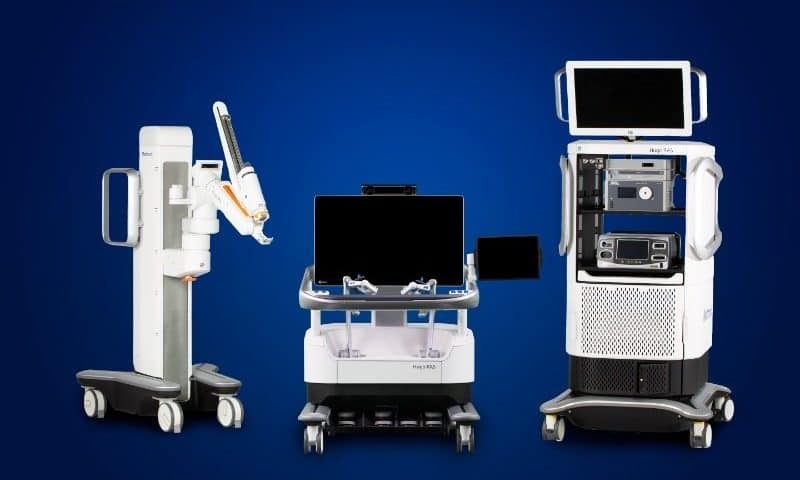
Medtronic has secured European approval for its Hugo surgical robot, clearing the way for the system to make its continental debut in multiple countries.
Hugo performed its first human procedure this past summer—a minimally invasive prostatectomy in Santiago, Chile. It has since expanded to Latin America, with a series of initial gynecological surgeries completed in Panama City, Panama.
The robotic platform, made up of modular surgical arms on wheeled carts, has also checked off its first operation in the Asia-Pacific region, through a prostatectomy in Chennai, India. All of the company’s systems are linked to a global patient registry, which tracks outcomes and feeds that data back into the platform.
Now in Europe, the CE mark covers urologic and gynecologic procedures, such as hysterectomies and the removal of uterine fibroids. Medtronic says the green light unlocks access to about half of all robotic surgeries being performed today.
“This day has been a long time coming, not just for Medtronic, but for the surgeons and hospital leaders who have partnered with us on this journey to bring the benefits of robotic-assisted surgery to more patients around the world,” Megan Rosengarten, president of Medtronic’s surgical robotics business, said in a statement.
The CE mark largely follows a timeline first laid out by the company in late 2019, which had its sights set on Europe before chasing regulatory approvals from the FDA and in broader soft-tissue surgery indications.
The Hugo system is designed to provide a lower barrier to entry for hospitals looking to expand their reach in robotic surgery. Compared to platforms such as Intuitive Surgical’s cornerstone Da Vinci system—a large, multiarm machine that’s typically installed as the centerpiece of an operating room—Hugo can be customized with up to four independent arms, and rolled to different locations in a hospital when needed.
Medtronic’s ultimate goal is to offer robot-assisted procedures at a cost similar to manual laparoscopy. The company estimates that only about 3% of procedures today are done robotically, while the majority are still performed through open surgery.
“Robotics and artificial intelligence are the undeniable future of healthcare, with incredible potential to not only advance patient care but increase access to these benefits,” said Rob ten Hoedt, Medtronic’s Europe, Middle East and Africa regional president. “We’ve had strong interest from leading surgical centers across Europe and expect to move quickly with multiple installations in several countries.”
To start, clinicians will receive hands-on training at Medtronic surgical centers including through two flagship sites located at the Orsi Academy in Ghent, Belgium, and the IRCAD laparoscopic training center in Strasbourg, France.
The Hugo system also incorporates 3D visualization and cloud-based video capture technology through the company’s Touch Surgery platform, used to help train surgeons and students outside the OR.
In Europe, Hugo will face off against CMR Surgical’s Versius robot, featuring its own cart-based, modular design. Versius first scored a CE mark in 2019 and is currently undergoing FDA review, with approval possible this year.
In preparation, the former Fierce 15 winner—previously known as Cambridge Medical Robotics—banked a massive $600 million venture capital round this past June, one of the largest ever in the medtech industry, and recently announced plans to build a new global manufacturing hub in the U.K. to meet projected demand.