As ventilators and other respiratory devices became more crucial than ever throughout the COVID-19 pandemic, so too did any safety issues embedded in the machines become more apparent.
Following in the footsteps of Philips and Covidien—both of which have initiated recalls in the last year within their respiratory portfolios—Dräger is now recalling a device of its own that’s used alongside mechanical ventilators.
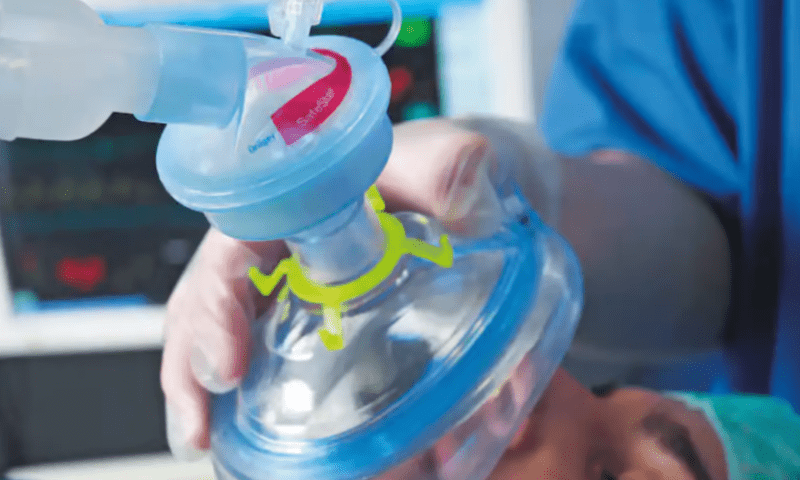
The safety notice concerns the German devicemaker’s SafeStar 55 breathing system filters, which connect to hospital-grade ventilators to prevent bacteria, small particles and other contaminants from entering the airflow of a patient receiving anesthesia or breathing assistance.
As with the Philips and Covidien recalls before it, Dräger has also been given the FDA’s most serious designation, as a Class I recall.
In total, Dräger has recalled 35,950 filters distributed in the U.S. during about a two-month period between August 18 and October 12 of last year.
The company began the recall in May after discovering that during that period, some defective filters may have accidentally been distributed rather than destroyed. Dräger attributed the mistake to an error in its manual inspection process.
The defective filters include some that may be partially obstructed. If connected to a ventilator, that could prevent oxygen from flowing properly to a patient, potentially leading to hypoxia, or a lack of oxygen, which can progress into organ damage and even death if not caught and treated in time. To date, Dräger has received one complaint and one report of injury linked to the affected filters, with no deaths reported.
Healthcare providers who use the SafeStar 55 breathing system filters should check their stores of the devices for any labeled with lot number LT2103. Those filters should be quarantined immediately and will be exchanged by Dräger at no cost.
The safety event is Dräger’s first Class I recall in over three years. The last arrived at the end of 2018 when the company recalled a total of 1,200 anesthesia breathing circuit kits, which are single-use devices designed to conduct respiratory gases between an anesthesia machine or ventilator and a patient, used either with or without a water trap to collect condensation.
In that case, certain VentStar breathing circuit kits and ID Circuit water traps were recalled after Dräger found that some of the systems had been incorrectly pre-assembled. Incorrect assembly could cause a breathing hose to short circuit, preventing a patient from receiving needed ventilation or anesthesia.