The already sizable list of recent Class I recalls of ventilators—linked to issues that have emerged en masse as the COVID-19 pandemic made the breathing support machines more essential than ever—just got longer.
In the last year alone, Philips, Covidien and Dräger have all recalled ventilators or related accessories from their respiratory portfolios. Latest to join the queue is GE Healthcare, which issued a safety alert in April for nearly 118,000 batteries used to provide backup power to some of its ventilators.
This week, the FDA gave the recall its most serious rating, a Class I label, which links a high risk of serious injury or death to a specific device flaw.
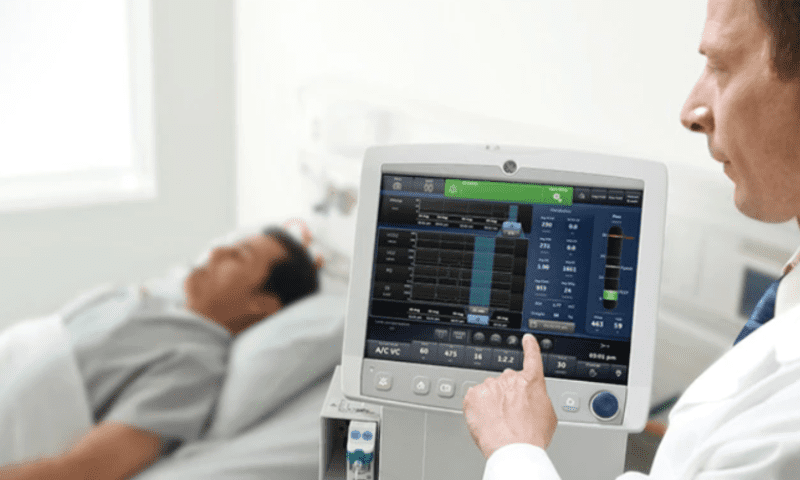
In this case, the flaw is related to batteries used in GE’s Carescape R860, Engström Carestation and Engström Pro ventilators. All of the models primarily run on power from a wall outlet but can temporarily operate using backup battery power during patient transports, power outages and other situations when AC power isn’t available.
GE has identified an issue with some of the batteries manufactured since April 1, 2019, in which they run out of power before they’re supposed to. The medtech giant is still investigating the root cause of the issue.
If the loss of power occurs while a ventilator is running on the backup battery power, it could stop a patient from receiving necessary oxygen and breathing support, potentially leading to severe injuries or death. To date, according to the FDA, GE has received a total of 1,553 complaints related to the ventilators’ backup batteries. No injuries or deaths have been reported.
While it continues to search for the cause of the failing batteries, GE is recommending that healthcare providers immediately run a battery performance test on their ventilators, then repeat the process every three months and before using devices that have been in storage for more than three months. They should also keep ventilators plugged in at all times, even while in storage, to prevent battery degradation, and make sure to replace batteries when necessary—at least every three years.
If a respiratory machine must be unplugged and forced to rely solely on backup battery power, users should have an alternative ventilation device, such as a bag-valve mask, at the ready.
The recall spans more than 29,400 backup batteries distributed around the world for use with the Carescape R680 machines, plus more than 88,300 replacement backup batteries that were sold around the world to users of the Carescape machines and both Engström models.
According to the FDA, a total of 4,222 of the affected batteries were distributed in the U.S. alone between April 2, 2019, and April 18 of this year—the same day GE initiated the recall.