Nearly four years after securing its initial clearance from the FDA, an algorithm designed to watch for signs of hypercapnia in children in intensive care has broadened its scope.
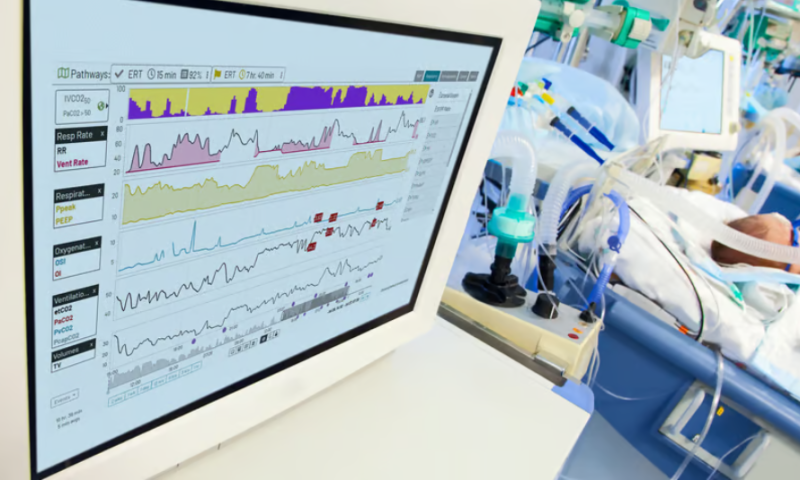
The IVCO2 Index was developed by Etiometry and named for its goal of detecting the inadequate ventilation of carbon dioxide. It uses artificial intelligence to monitor real-time patient data, sending out an alert if carbon dioxide levels are on track to rise too high in a mechanically ventilated patient, potentially leading to hypercapnic respiratory failure.
The AI algorithm was first cleared by the FDA at the end of 2019, with the agency’s go-ahead to monitor for hypercapnia risk in patients between the ages of 29 days and 12 years who weigh at least two kilograms and are on ventilators in an intensive care unit. That indication was slightly expanded last year, allowing the algorithm to be used from the day a patient was born.
Now, the technology’s brief has stretched even further, as Etiometry announced this week that the IVCO2 Index can now be used on newborns in the neonatal ICU who weigh under two kilograms.
Etiometry said its algorithm can help circumvent some of the current difficulties of monitoring this tiny and especially fragile patient population with standard tools like arterial blood gas testing and transcutaneous monitoring.
The IVCO2 index is displayed as a digital readout on Etiometry’s software platform that clinicians can refer to between blood gas tests to keep an eye out for signs of hypercapnia. The index increases in tandem with the risk of inadequate carbon dioxide ventilation—offering a prediction of whether a patient’s partial pressure of carbon dioxide will cross into the danger zone above 50 mmHg.
“The IVCO2 Index is a first-of-its-kind algorithm that increases the ability to detect hypercapnia risk without needing additional hardware attached to these fragile patients,” Dimitar Baronov, Etiometry’s chief technology officer, said in the announcement. “It allows clinicians to prioritize care for patients who need it most.”
The latest FDA clearance is Etiometry’s ninth since its 2010 founding and its second this year alone.
In January, the Boston-based company marked its first foray into older populations in the U.S. as its long-standing IDO2 Index was cleared by the FDA to detect the risk of inadequate oxygen delivery in adult patients in the ICU.
The IDO2 Index has been available for use in pediatric populations in the U.S. since 2016. Like the IVCO2 index, it’s digitally displayed on the Etiometry platform and works by using AI to analyze the vast amounts of patient data continuously collected in the ICU around the clock. In this case, however, the algorithm looks for instances in which the amount of oxygen in a patient’s blood is dropping, with an aim of sending out an alert before it reaches the hypoxic respiratory failure phase.