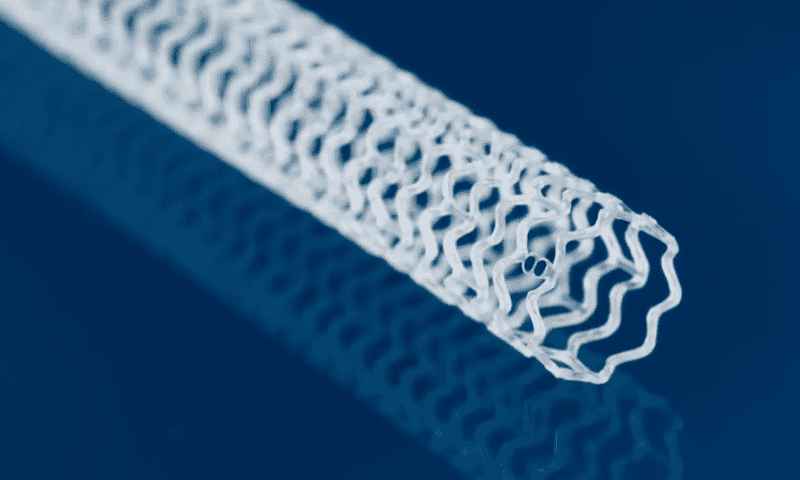
Abbott has delivered a landmark trial of a drug-eluting, bioresorbable scaffold implant designed to support a reopened leg artery, among people facing the possibility of amputations below the knee due to severely blocked blood flow.
People with chronic, limb-threatening ischemia in the lower limbs have few treatment options; the advanced form of peripheral artery disease currently has no drug-coated balloons approved for treatment in the U.S., nor any drug-eluting or bare-metal stents.
Abbott’s approach involves placing the Esprit BTK implant within the artery after the current standard-of-care balloon angioplasty procedure, to help hold it open as the vessel heals. According to the company, the scaffold is made of a naturally dissolving material that is absorbed by the body within about three years, similar to certain sutures. It also releases a dose of everolimus over time, similar to Abbott’s metal drug-eluting stents.
In the randomized LIFE-BTK study, presented as a late-breaking trial this week at the annual Transcatheter Cardiovascular Therapeutics conference in San Francisco, the Esprit implant bested its primary endpoints in comparison to treatment with standard balloon angioplasty alone.
In 261 participants, the study showed the addition of Esprit BTK reduced the rates of blood vessel re-narrowing after the procedure by 25.8% and delivered a 14.2% improvement in maintaining the open passageway.
Patients who received the scaffold also saw significantly fewer clinical events—such as amputations starting above the ankle, total blockage of the targeted vessel or the need for a repeat procedure within one year. The study also tracked major limb interventions, including surgical bypass grafting or mechanical blood clot removals.
After 12 months, 74% of patients treated with Esprit BTK had zero of these clinical events, compared to only 44% of those in the angioplasty arm, while safety measures between the two groups were similar. The pivotal results were simultaneously published in the New England Journal of Medicine.
“The LIFE-BTK trial data underscores the profound impact that Esprit BTK could have for millions with PAD,” Jennifer Jones-McMeans, divisional VP of global clinical affairs for Abbott’s vascular business, said in a statement.
“With angioplasty, multiple interventions are all too common. The results from this trial demonstrate a compelling and meaningful development in clinical outcomes, ultimately helping people have less adverse events and reinterventions, enhancing their quality of life,” Jones-McMeans added.
Abbott said it plans to submit the Esprit BTK for FDA review.
The company previously pursued bioresorbable stents for the coronary arteries. It received an FDA approval in 2016 for its everolimus-eluting Absorb heart implant, but a subsequent agency analysis found a higher rate of major cardiac side effects when compared to newer versions of drug-eluting metal stents. The company pulled Absorb from the market in 2017.