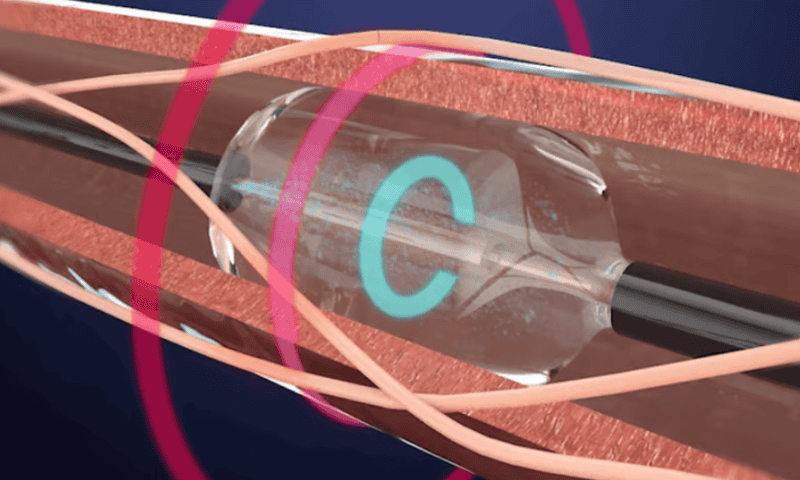
Recor Medical has crossed the FDA’s finish line with its renal denervation approach to tackling high blood pressure that hasn’t responded to medications or changes in a person’s lifestyle.
It’s been a long road for the company and its Paradise system, which began development in 2009. The catheter-based device uses ultrasound energy to generate heat and aims to treat hypertension by disabling the nerves that line the main blood vessels feeding into the kidneys.
Heightened activity in these nerves is believed to push the brain to increase the body’s blood pressure and with it, a person’s lifetime risks of heart disease and stroke.
Recor, a division of Tokyo-based Otsuka, had previously seen a clinical trial in Japan and Korea fail to achieve its primary endpoint in 2021; the company ascribed the miss to muddied data caused by inadequately controlled use of blood pressure medications.
But the company turned things around in the time since, with a randomized, sham-controlled study last year demonstrating that Paradise could cut down daytime systolic blood pressure within two months following the one-time procedure, shaving nearly 8 mmHg off the reading’s top number on average.
Most recently, Recor spotlighted a combined analysis from three clinical trials totaling 500 patients at the annual Transcatheter Cardiovascular Therapeutics conference in San Francisco showing it could maintain reductions for six months with fewer patients being prescribed additional antihypertensive drugs.
But the clearest signal that Recor was on track for an FDA approval was an August meeting of an agency advisory committee, which evaluated data from Paradise’s studies as well as the promise of its potential competitor, the Symplicity Spyral developed by medtech giant Medtronic.
Both companies plan to compete in what they have estimated to be a multibillion-dollar market, and both have already secured regulatory green lights in Europe. Over a two-day meeting, the FDA’s advisory panel voted 8-3 that Paradise had demonstrated its efficacy and agreed 10-2 that its benefits outweighed the risks.
Medtronic saw a much slimmer margin: Symplicity Spyral’s ablative approach received a 7-6 vote in favor of effectiveness and a 6-6 tie on its risk/benefit profile, with one abstention. The panel’s chair broke the deadlock with a vote against recommending for approval. Both devices were unanimously considered to be safe.
“Recor is leading the way in bringing an innovative solution to clinicians and their patients struggling to control blood pressure,” Recor President and CEO Lara Barghout said in a statement. “This FDA approval is the culmination of years of technical research and rigorous clinical studies.”
“We are grateful to the patients who participated in the studies and to the clinical trial investigator teams whose diligence and dedication made FDA approval possible,” Barghout added. “We look forward to making this technology available to physicians and their patients nationwide.”