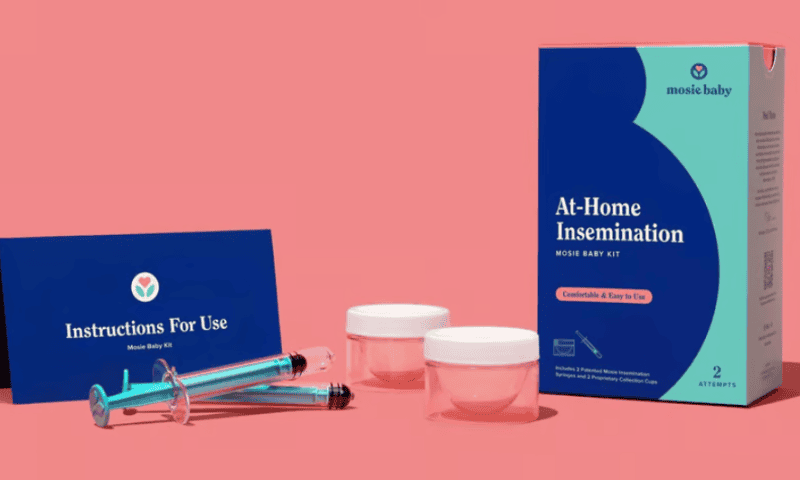
The FDA has cleared its first over-the-counter kit for at-home intravaginal insemination—opening up access to fertility care for people unable to conceive through intercourse, without requiring a clinic appointment.
Developed by Mosie Baby, the $130 kit contains two pairs of collection cups and specially designed syringes, built to comfortably deliver semen into the vaginal canal near the opening of the cervix.
According to the company, the agency’s 510(k) clearance found its intravaginal methods to be substantially equivalent to devices used in intrauterine insemination performed by a clinician, a procedure commonly known as IUI.
Leading up to its regulatory green light, Mosie Baby said its equipment passed internal testing including sperm survival assays and biocompatibility exams. The kit can be used with fresh or cryogenically frozen donor semen.
The proprietary cups and syringes are designed with rounded-off edges inside and out, meant to minimize waste while drawing and delivering a sample. The syringes also feature a wider, oval-shaped opening for a more gentle flow than traditional syringes.
According to the Texas-based Mosie Baby, the at-home insemination kit will first be available through CVS, Walmart, Amazon and Optum, as well as the company’s website. It said it plans to launch with additional retailers and healthcare partners in 2024.