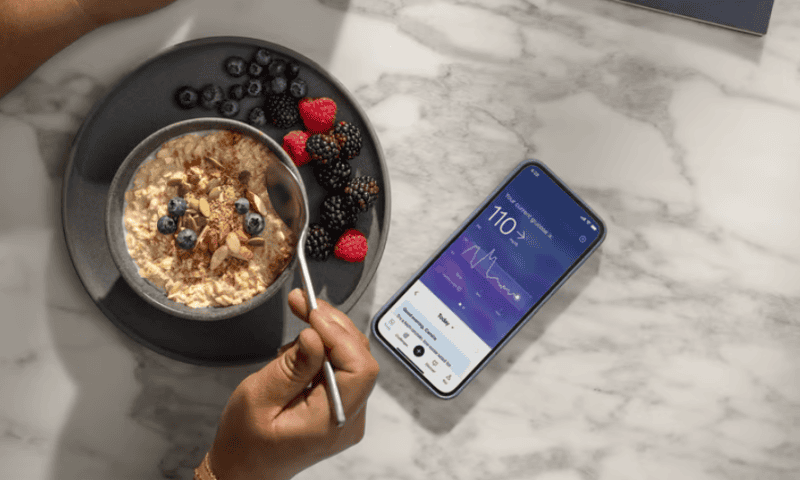
With the rollout of its new over-the-counter continuous glucose monitor, Abbott aims to help its customers speak the hidden language of their bodies’ response to food, exercise and stress.
The wellness-focused Lingo received the FDA’s blessing earlier this year, as one of the company’s first wearable blood sugar trackers to be offered without a prescription. The device is now making its U.S. commercial debut; it’s available for adults that are not taking insulin, but who Abbott says may still be interested in pursuing healthier habits backed up by personal data.
“Glucose is a powerful signal of your body’s unique response to food and lifestyle,” Olivier Ropars, divisional VP of the company’s Lingo business, said in a statement. “Abbott’s Lingo tracks your glucose 24/7, translating the data into insights and bridging the gap between traditional healthcare and preventative measures.”
The Lingo is built off of Abbott’s FreeStyle Libre CGM, used by people with Type 1, Type 2 and gestational diabetes. It received an agency clearance in June alongside the Libre Rio—a similarly stepped-down, over-the-counter version of the company’s platform, instead aimed at adults with Type 2 diabetes that are not taking insulin.
Like its sibling sensors, the Lingo is worn on the back of the upper arm. The device lasts for up to 14 days, and charts a person’s real-time glucose readings and blood sugar spikes. Abbott is offering the system in three different packages, based on how long a user may want to log their data.
That includes a two-week stint, with one sensor available for $49; a four-week package of two for $89; and a three-month option containing six for $249.
Lingo’s launch comes quick on the heels of Dexcom’s over-the-counter entrant, the Stelo, which began shipping to customers late last month. Also pitched as a health-and-wellness device, a two-pack of that CGM is priced at $99, for a total wear time of up to 30 days. Dexcom is also offering a recurring subscription that delivers two Stelo sensors, at $89 per month.
And as CGMs begin to make headway in expanding beyond Type 1 diabetes—including to the millions of people with Type 2 diabetes who may or may not take insulin—automated insulin pumps are starting to follow suit as well.
In late August, the FDA handed a new clearance to Insulet’s Omnipod 5, opening up its use to an estimated 6 million additional people, including about 2.5 million with Type 2 diabetes who are taking multiple injections per day to help manage their blood sugar. And this week, embecta received a green light for its first patch pump, in both Type 1 and Type 2 diabetes.