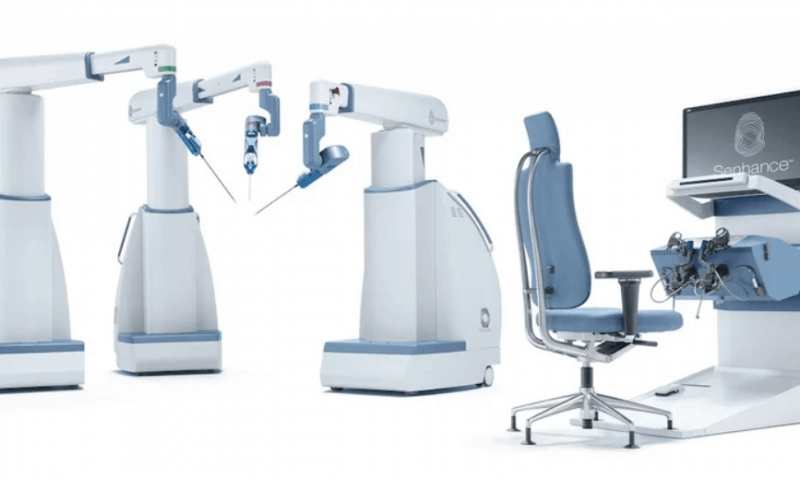
Shortly after TransEnterix changed its name to Asensus Surgical late last month—calling the rebranding a signal of its plans to bring artificial intelligence to the operating table—the company has secured a new, expansive FDA clearance for its robotic system in general surgery.
Its Senhance laparoscopic system provides eye-tracking camera controls, haptic feedback and 3D visualization, paired with instruments as small as 3 millimeters, for minimally invasive procedures.
“The expansion into general surgery for the Senhance Surgical System is a major milestone for the growth and clinical applicability of our technology,” said Anthony Fernando, Asensus’ president and CEO.
“The indication expansion allows Senhance to be used in many high-value, complex reconstructive surgeries such as those used to treat reflux and obesity,” Fernando said—estimating that, in total, the system can now be used in more than 2.7 million surgeries that are performed in the U.S. each year, spanning nearly the entire abdomen, in addition to its previous clearances for gynecologic surgeries.
Currently, the Senhance system is used by about 100 surgeons globally, the company said, with more than 4,000 procedures performed in different surgical specialties. The end goal is to wield machine learning and computer vision technologies to help bring the lessons learned in one operating room and digitally transfer them to another.
“As the company evolves from a robotics company to a digital surgery company, the rebrand better reflects our vision and we know that Asensus has the technology, the team, and the opportunity to create a new paradigm in best surgical practices and techniques we call performance-guided surgery,” Fernando said.