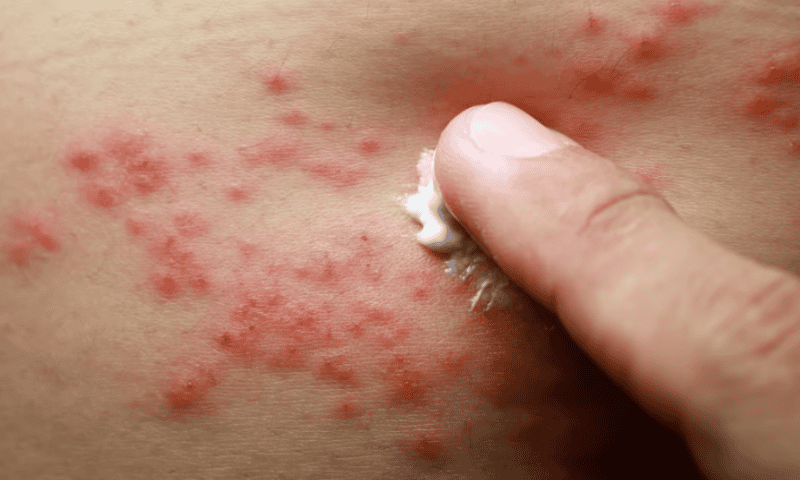
Undeterred by the FDA’s rejection earlier this month, Eli Lilly has posted long-term data demonstrating its atopic dermatitis treatment lebrikizumab can provide sustained skin benefits over two years.
The data comes from the ADjoin trial, which took on patients who had already seen clinical benefits after 16 weeks in either the ADvocate 1, ADvocate 2 or ADhere phase 3 trials. Participants received a 250-mg injection of the anti-IL-13 antibody either every two weeks or monthly.
Of the 99 patients who received a monthly dose after moving from the ADvocate trials, 76% were assessed as having clear or almost clear skin after two years, compared with 86% of the 82 patients from those trials who received a dose every two weeks. The proportions who achieved at least a 90% improvement on the Eczema Area and Severity Index (EASI) were 83% and 82%, respectively.
A total of 90% and 100%, respectively, of these cohorts saw a four-point or larger improvement in their itching, known as pruritus, according to Lilly’s data table.
Patients who moved across from the ADhere trial recorded similar responses, with 79% of the monthly dose cohort and 84% of the twice-weekly cohort assessed as having clear or almost clear skin after two years. Those proportions showing at least 90% EASI improvements were slightly more varied, at 72% and 85%.
The drug’s safety was consistent with previous studies in moderate to severe atopic dermatitis, and no new safety signals were observed, the Big Pharma said. The findings, which are being presented at the Clinical Dermatology Conference in Las Vegas, “reinforce the strong efficacy and safety profile of lebrikizumab seen in the other phase 3 atopic dermatitis trials,” Lotus Mallbris, M.D., Ph.D., Lilly’s senior vice president of global immunology development and medical affairs, said in a release.
“These data also further our understanding of the long-lasting benefits of lebrikizumab as a potential first-line biologic treatment for patients,” Mallbris added. “We look forward to working with global regulatory authorities to bring this important medicine to patients.”
Almirall owns the European rights to lebrikizumab, and the company’s chief scientific officer, Karl Ziegelbauer, Ph.D., said today’s readouts “bring us one step closer to offering a first-line biologic treatment option” for patients with moderate to severe atopic dermatitis.
Lebrikizumab hasn’t had much luck with regulators so far. Earlier this month, the FDA rejected the drug over findings uncovered during an inspection of a third-party contract manufacturing organization.
The agency’s complete response letter raised no issues with lebrikizumab’s clinical data package, nor did the FDA flag problems with the med’s label or safety, Lilly said at the time. But it marked Lilly’s third rejection this year, following slapdowns of mirikizumab for ulcerative colitis and donanemab’s bid for an accelerated approval in Alzheimer’s disease.