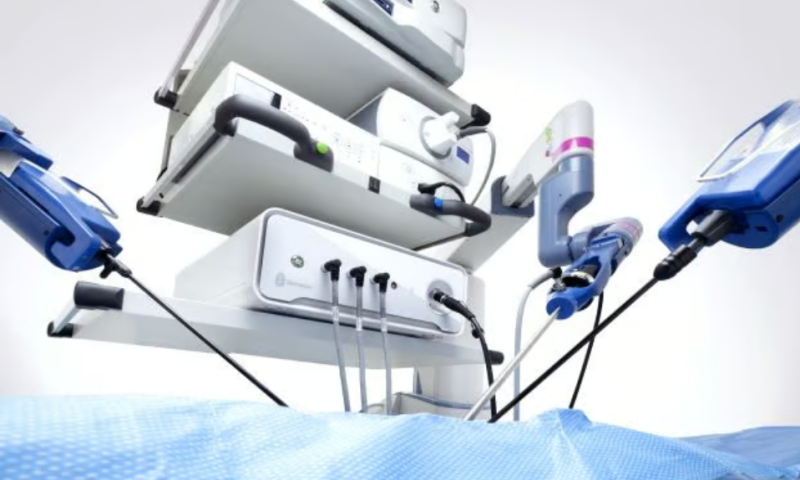
A malfunction that could cause Asensus Surgical’s robotic system to move unexpectedly has received the most serious classification from the FDA.
The company’s laparoscopic Senhance system, with its multiple independent robotic arms, carries clearances for minimally invasive general surgery as well as gynecological procedures alongside its machine-vision-powered camera software.
According to the FDA, the platform’s laparoscope instrument actuator could potentially begin rotating continuously after being disconnected from its digital teleoperation remote control systems. Asensus sent a letter to its customers Sept. 15 alerting them to the issue and urging them to stop using Senhance until the company could deliver a software update.
“There has been no patient impact or harm that has occurred due to this issue, however, the potential for critical tissue trauma is possible,” the FDA said in its Class I recall notice, adding that Senhance also has emergency stop capabilities. The agency said the malfunction could affect as many as 21 placed systems.
Earlier this week, Asensus reported its third-quarter results, noting that it has sold five new Senhance systems so far this year—including one in the U.S., two in Japan, one in Germany and one in a pediatric hospital within what the company described as the “Commonwealth of Independent States region,” which includes Russia and several countries of the former Soviet Union.
The company also recorded 17% growth in its number of quarterly surgical procedures compared to the same period in 2022, for a grand total of more than 2,700 globally as of the end of September.
However, that procedure growth did not translate into cash: Asensus collected $1.1 million in revenue for the third quarter, down from $2.6 million the year before. Operating expenses, meanwhile, totaled $18.5 million, up from 2022’s $17.2 million, as the company works to develop its upcoming minimally invasive Luna surgery system.
Asensus said it plans to complete Luna’s integrated system testing and begin preclinical evaluations before the end of the year. The company also recently inked a design and production deal with contract manufacturer Flex.