BD is building on its goal of providing patients with a “one-stick hospital stay” with a new FDA clearance for painless blood collection hardware that works through already placed IV lines.
The company first picked up the needle-free blood draw system through its 2021 acquisition of Velano Vascular, maker of the PIVO device, which threads a small, flexible catheter through an IV line and into the veins of the arm. This allows it to collect samples away from any contaminating debris, medications or fluids while being able to fill as many tubes as needed over the course of a patient’s visit.
PIVO first collected an FDA green light in 2017 for a version of the device compatible with traditional short peripheral IV catheters. The latest model, dubbed the PIVO Pro, expands the design to work with integrated and long catheters—such as BD’s Nexiva closed system, which can be used for up to six days.
“This new solution helps to reduce unnecessary and repeat needlesticks in the hospital while elevating clinical outcomes, improving workflow and creating a better experience for clinicians and patients,” BD’s worldwide president of medication delivery, Eric Borin, said in a statement.
According to BD, the combination of PIVO and its IV access taps have also shown reductions in sample errors resulting in redraws as well as complications that can lead to unnecessary procedures and line replacements.
The company estimates more than 3.5 million PIVO procedures have been completed in the U.S. to date, with peripheral IV insertions and blood collections being two of the most common events of a patient’s hospital experience.
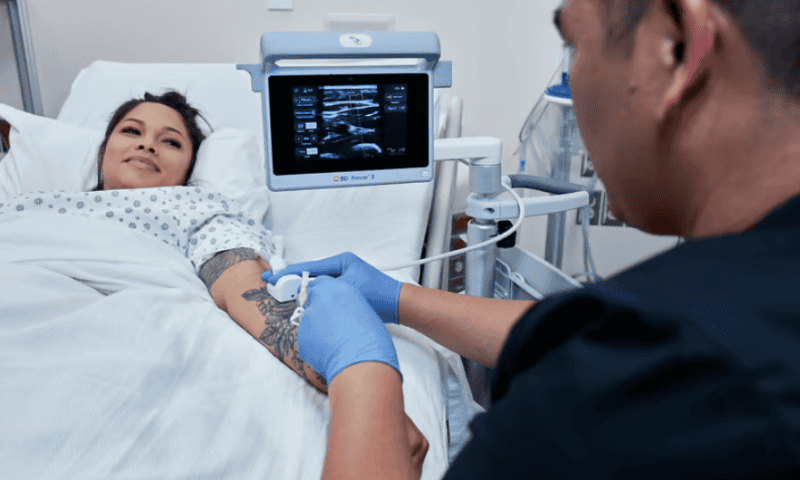
The PIVO system serves as one of three pillars of BD’s “one-stick hospital stay” mantra. The others include maintaining the line during the stay so it does not have to be replaced and placing it correctly the first time—the latter of which is supported by the company’s launch earlier this year of an ultrasound probe aimed at finding the perfect vein for an IV.
The company’s Prevue II system, announced in April, provides a view under the skin and can track the depth of a magnetically tracked needle in real time.