Blood cancers such as leukemia are caused by genetic changes in the blood-forming stem cells of the bone marrow. Scientists at the University Medical Center Mainz have now shown how chronic inflammation can alter the bone marrow in people with age-related blood stem cell mutations at very early stages of the disease, thereby promoting its development.
They found that the interaction of inflammatory tissue stem cells and certain immune cells triggers self-reinforcing inflammatory processes in the microenvironment of the bone marrow, which impair normal blood formation. The findings, published today in the journal Nature Communications, could form the basis for the development of novel therapies for the early treatment of blood cancer.
Every second, human bone marrow produces several million new blood and immune cells. This continuous cell renewal is based on the interaction between blood-forming (hematopoietic) stem cells, or HSCs for short, supporting connective tissue cells (stromal cells), and molecules or cells that control the immune system (immune regulators).
The bone marrow microenvironment
The microenvironment of the bone marrow, also known as the bone marrow microenvironment, is essential for the formation of blood cells. It enables the exchange of signals between cells and thus influences the growth of both healthy and genetically altered, potentially disease-causing cells. Despite its great importance for blood formation, little is known about how the microenvironment of the bone marrow contributes to the development of blood cancers.
An international research team led by Dr. Borhane Guezguez, working group leader at the Department of Internal Medicine III of the University Medical Center Mainz, and Dr. Judith Zaugg, working group leader at the European Molecular Biology Laboratory (EMBL) in Heidelberg and professor at Basel University, has now discovered that chronic inflammatory processes in people with blood stem cell mutations can lead to cellular changes in the bone marrow microenvironment long before disease symptoms appear.
Genetic mutations and disease risk
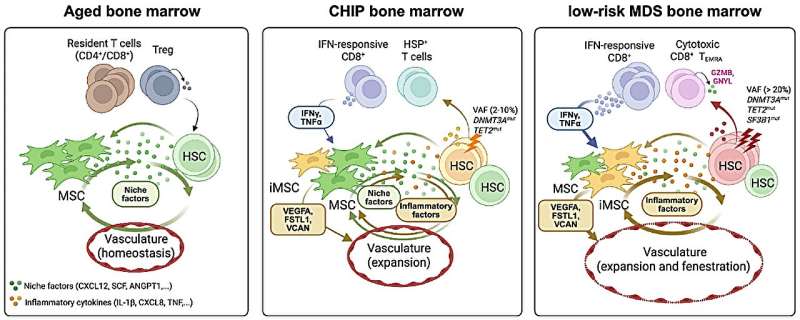
The scientists examined the microenvironment of the bone marrow of subjects with specific genetic changes in their hematopoietic stem cells: Clonal hematopoiesis of indeterminate potential (CHIP) can develop as part of the aging process and occurs in about 10% to 20% of people over the age of 60 and in almost 30% of people over the age of 80. Although CHIP is asymptomatic, it increases the risk of blood cancer tenfold, doubles the risk of cardiovascular disease, and is associated with increased mortality.
Myelodysplastic syndromes (MDS) are a group of disorders characterized by impaired blood cell production and progressive bone marrow failure. MDS also occurs primarily in older age groups—up to 20 out of 100,000 people over the age of 70 are affected. In up to 30% of cases, MDS develops into acute myeloid leukemia (AML), an aggressive and often fatal form of blood cancer.
Inflammatory cells disrupt healthy blood formation
In the microenvironment of the bone marrow of subjects with CHIP and MDS, the researchers discovered a group of inflammatory connective tissue stem cells (inflammatory mesenchymal stromal cells) that displaced the normal connective tissue stem cells in the bone marrow.
Unlike healthy stromal cells, the inflammatory cells release large amounts of signaling molecules that attract and activate certain immune cells that respond to the body’s own protein interferon, a component of the immune system. These interferon-responsive T cells further intensified the inflammatory processes and disrupted normal blood formation.
Implications for early detection and prevention
“Our research shows that the microenvironment of the bone marrow actively influences the earliest stages of blood cancer development,” emphasizes Dr. Guezguez, last author of the recently published study. “Thanks to advances in genetic analysis, we can detect precursors of blood cancers years before symptoms appear.
“Our new findings on the interactions between stromal and immune cells could therefore form the basis for preventive therapies for CHIP or MDS that target the bone marrow microenvironment and prevent the disease from progressing before leukemia develops. The distinct molecular signatures of inflammatory mesenchymal stromal cells and interferon-responsive T cells could also serve as biomarkers to identify at-risk individuals long before clinical symptoms appear,” explains Dr. Guezguez.
According to the scientists, the research contributes to a better understanding of so-called “inflammaging,” a chronic, mild inflammation that underlies many age-related diseases—from cancer to cardiovascular and metabolic diseases.