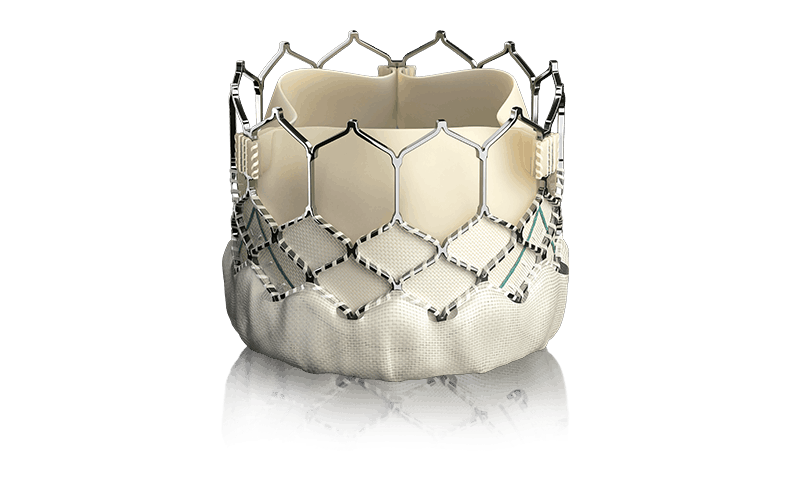
A five-year study by Edwards Lifesciences demonstrated no significant differences in the number of deaths or disabling strokes among patients who received a transcatheter aortic valve replacement, or TAVR, compared to those who received a new valve through heart surgery.
However, the study did show that patients in the TAVR group had more frequent rehospitalizations, reinterventions and leak complications in the years following the procedure.
Researchers also noted that all-cause mortality rates and disabling strokes were higher in the TAVR group between the second and fifth years following the procedure, potentially due to the increased number of leaks or untreated coronary artery disease.
The study enrolled more than 2,000 patients at intermediate risk for surgery and with severely narrowing and calcified aortic valves. They were randomly assigned to have minimally invasive placements of Edwards’ Sapien XT heart valve or traditional surgical valve replacement methods.
Patients were stratified further based on the route the implant took to reach the heart—either by a catheter inserted into the thigh’s femoral artery or through the chest wall and via transapical or transaortic approaches. The study was published in The New England Journal of Medicine.
After five years of tracking clinical, echocardiographic and other outcomes, researchers found that improvements in health status between the two study arms were similar.
The transfemoral route provided no significant differences in rates of mortality or disabling strokes, with 44.5% compared to the surgical cohort’s 42%. But, at the same time, the study’s transthoracic group showed higher rates, at 59.3%, compared to surgery’s 48.3%.
Out of all patients who underwent TAVR, 33.3% showed signs of mild paravalvular aortic regurgitation, versus surgery’s 6.3%. One-third of TAVR patients also saw repeat hospitalizations, while only about a quarter of surgery patients did, and aortic valve reinterventions were more common, at 3.2% compared to 0.8%, respectively.
One limitation of the study is that it employed the balloon-expandable Sapien XT valve, which is no longer in clinical use. The current Sapien 3 includes an external sealing skirt and is sized using CT imaging prior to the procedure; Edwards said it has seen fewer leaks compared to previous device generations in separate studies.
In August 2019, the FDA granted Edwards, as well as its competitor Medtronic, expanded approvals for their TAVR systems, broadening their use to patients with a low risk of complications from open heart surgery.
In clinical studies evaluating valves from both companies, TAVR patients showed lower rates of all-cause mortality or disabling stroke at 30 days, with higher blood flow, as well as shorter hospital stays and discharges to home or self-care. Edwards’ Sapien family of heart valves received FDA approval for intermediate-risk patients in 2016.