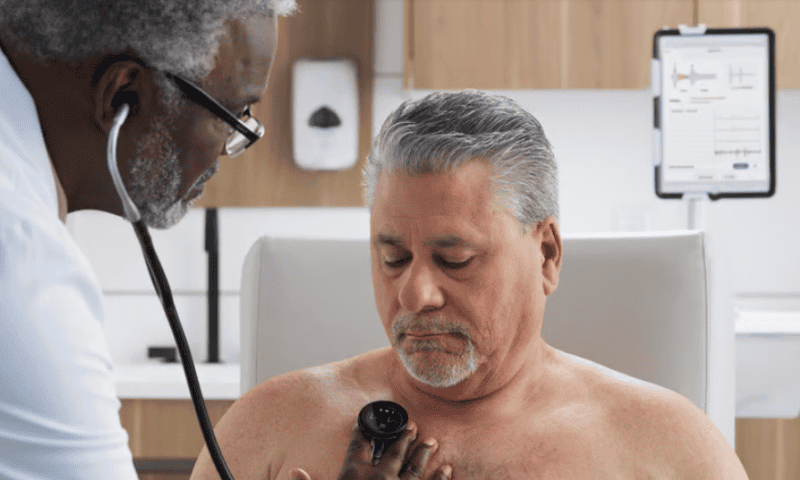
Eko Health has secured a groundbreaking clearance from the FDA for an artificial intelligence program that can capture a key indicator of heart performance through its digitally enhanced stethoscopes.
Developed in collaboration with the Mayo Clinic, the software can detect cases of low ejection fraction—a primary sign of heart failure, where the beating organ is unable to efficiently pump out blood to the rest of the body with each heartbeat.
Heart failure is typically split between patients with reduced ejection fraction, a condition known as HFrEF, and those with preserved levels, with a complete diagnosis typically requiring a specialized ultrasound exam. Eko estimates more than 3 million people in the U.S. may have HFrEF, with many going untreated.
“The ability to identify a hidden, potentially life-threatening heart condition using a tool that primary care and subspecialist clinicians are familiar with—the stethoscope—can help us prevent hospitalizations and adverse events,” the Mayo Clinic’s cardiovascular medicine department chair, Paul Friedman, M.D., said in a statement.
“Importantly, since a stethoscope is small and portable, this technology can be used in urban and remote locations, and hopefully help address care in underserved areas,” Friedman added.
Eko’s digital devices include sound amplification and noise cancellation, as well as the ability to simultaneously record a three-lead electrocardiogram. The company said a 15-second reading during a routine physical exam is enough to spot the signs of low ejection fraction.
With the agency’s green light, the Low EF AI program will be added to Eko’s Sensora digital platform, which also includes FDA-cleared algorithms for detecting atrial fibrillation and structural heart murmurs.
“The stethoscope, the most recognizable symbol of healthcare, touches the lives of an estimated one billion people around the globe every year,” said Eko co-founder and CEO Connor Landgraf. “With Eko’s Low EF AI, we’ve transformed the icon of medicine into an AI-powered heart failure early detection tool that can help improve access to care for millions of patients, at a fraction of the time and cost of echocardiography.”
According to Eko, its Low EF AI was trained on a data set of more than 100,000 pairs of electrocardiograms and ultrasound exams, demonstrating a 74.7% sensitivity and 77.5% specificity in its ability to differentiate reduced and normal ejection fraction.
Meanwhile, an independent evaluation by Imperial College London, published in The Lancet Digital Health in early 2022, posted 84.8% sensitivity and 69.5% specificity across more than 1,050 patients in real-world settings.