Intellia Therapeutics proved last year that gene editing can work in humans. But a lingering question was, for how long? Now, the biotech has the data to begin to answer that question.
In a hotly anticipated readout, Intellia is presenting additional data from the phase 1 trial for NTLA-2001 in transthyretin, or ATTR, amyloidosis during an investor event today. The company is filling in some pieces that were missing when initial data in June 2021 showed the CRISPR gene editing therapy significantly reduced a known biomarker of the disease.
Intellia has confirmed in interim data that the reduction in serum levels of transthyretin, or TTR, was maintained in patients after follow-up that ranged from two months to 12 months post-treatment. The company also said NTLA-2001 spurred a greater reduction in serum with a higher dose. Both are key pieces of data investors were eagerly awaiting. Plus, all this was achieved without any new concerning safety signals.
“What we show is that pretty much what you get on Day 28 is what you’re going to have thereafter,” Intellia CEO John Leonard, M.D., said in an interview with Fierce Biotech.
NTLA-2001 works by deploying lipid nanoparticles to the liver to drop off a two-part genome editing system. The first part is a guide RNA specific to the disease-causing gene, and the second part is a messenger RNA—the tech made famous by COVID-19 vaccines—to encode the Cas9 protein and carry out precision editing.
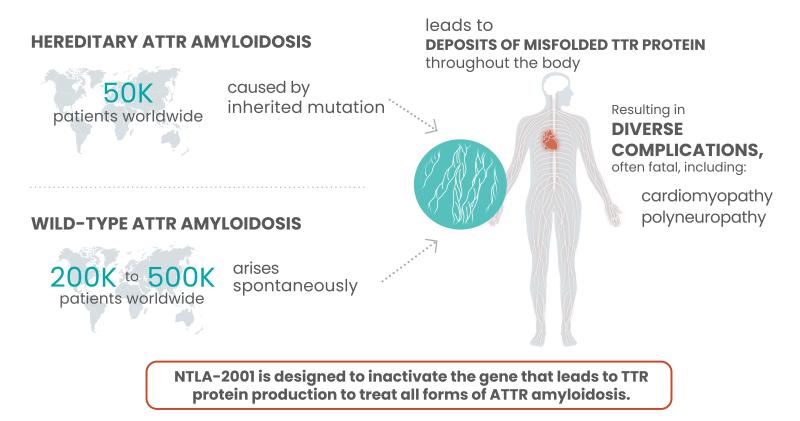
ATTR amyloidosis is a rare and fatal disease that occurs in people born with TTR gene mutations, which cause the liver to produce abnormal, often misfolded TTR proteins. The damaged proteins, which in a healthy form help carry thyroid hormone and vitamin A in the blood, build up in the body, causing a host of problems in the nervous system, heart and other organs.
The latest data show that the serum reduction seen at the initial Day 28 observation was sustained through the last check-in with each of the six patients who received a dose of 1.0 mg/kg. These patients experienced reductions higher than 80% and 90%, Intellia said. The initial data release last year showed a reduction of 87%.
Intellia has also settled on a dosing regimen for NTLA-2001 aimed at simplfying treatment going forward, Leonard said. Instead of weight-based dosing, the company will advance a fixed dose of 80 mg for all patients.
“You can simplify the regimen so you don’t have to worry about the pharmacy mixing up something incorrectly or a weight that’s wrong; it tends to just take a lot of complexity out,” Leonard said of the fixed-dosing regimen.
Intellia has found that while patients receiving the lower doses in the trial did have reduced serum levels, the effect was greater in the higher dose arms of the study.
As for the safety data, Leonard said one dose-limiting toxicity was observed in a single patient that experienced vomiting, which was rated as a grade 3 adverse event. This patient had prior medical history of vomiting due to gastroparesis, a common problem with ATTR amyloidosis in which the digestive system can’t clear food properly. The patient has since recovered, Leonard said.
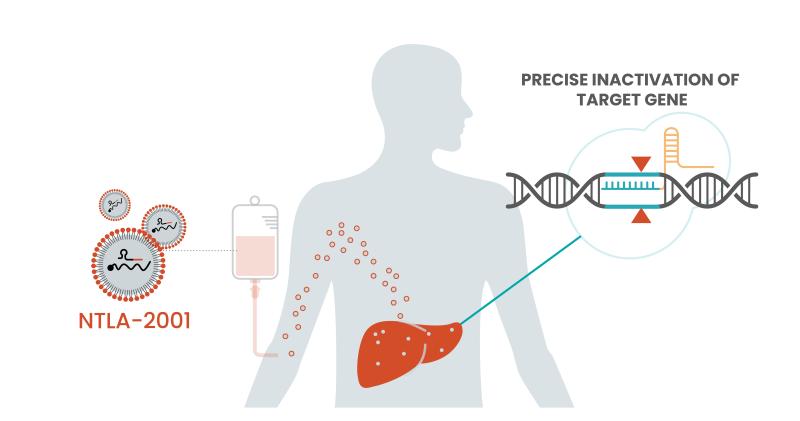
With some data now added to the durability question, Intellia could have itself a one-time treatment option for patients. ATTR amyloidosis is currently treated with one of three available treatments: Alnylam’s Onpattro, Ionis’ Tegsedi or Pfizer’s Vyndaqel. But Leonard said the company is more focused on the simple efficacy question along with whether this CRISPR therapy will benefit patients.
“The appeal is not one-and-done. That’s a nice secondary aspect of this thing. This is, first and foremost, an efficacy argument,” he said. “The idea being, from an amyloidosis point of view, you want to make the offending protein go as low as you possibly can.”
Intellia’s current studies are not geared to detect efficacy or patient benefit—that will come in more advanced testing later on—but TTR serum reduction is a known biomarker of the disease that signals a benefit. The actual benefits, i.e., relief from the symptoms of the disease, will not be immediate after treatment with NTLA-2001, Leonard said.
“What will a patient feel on Day 1? Nothing,” Leonard said, adding that that’s the goal: to kick off serum reduction without any adverse events.
But with the protein no longer getting deposited into the tissues, the hope is that the body’s natural processes will begin to clear out the buildup, he explained. Those natural processes are not fully understood.
“It’s, we think, in the realm of possibility that patients can actually improve relative to their baseline. That will take time,” Leonard said. “It’s not like the protein goes away, people get up and start playing tennis or something like that. You’ve got to let the process play out.”
Intellia plans to continue tracking the patients for the rest of their lives to record the durability data, so someday, we may have a more concrete understanding of the science behind ATTR amyloidosis.
As for next steps, Leonard and the team laid out the plan during Intellia’s fourth-quarter earnings Feb. 24 and during a presentation at the J.P. Morgan Healthcare conference in January. The company has initially targeted the polyneuropathy subtype of the disease, which shows up as nerve damage, in the study. But, now, a new patient group will be added to the early-stage study including those with the cardiomyopathy manifestation.
Even before seeing the updated data, RBC analyst Luca Issi, Ph.D., pointed to Intellia’s recent decision to build a new manufacturing facility as a vote of confidence for the therapy. Issi said Intellia’s therapy is likely to be more effective and convenient than the available silencer treatments like Tegsedi and Onpattro that have set the standard with serum reductions in the range of 70 to 80%.
Issi also pointed to early mouse data that showed gene editing was preserved when a portion of the liver was removed and allowed to grow back. Leonard said today’s results seem to confirm that preclinical testing.
Another thing that’s been confirmed for Leonard is the company’s approach with its broader pipeline, specifically treatments that belong in the in vivo category like NTLA-2001. One is NTLA-2002 in hereditary angioedema, which is in a phase 1 trial that has begun dosing patients, the CEO said. Data for that study will be presented later this year.
“The read-through should be very direct and quite immediate” on the pipeline, Leonard said of the latest results. Intellia has shown the lipid nanoparticles are getting to where they need to be in the liver, and that mechanism is the same across the in vivo program.
Intellia also has ex vivo candidates, which should be buoyed as well by the latest ATTR amyloidosis data, but to a lesser extent, Leonard said.

