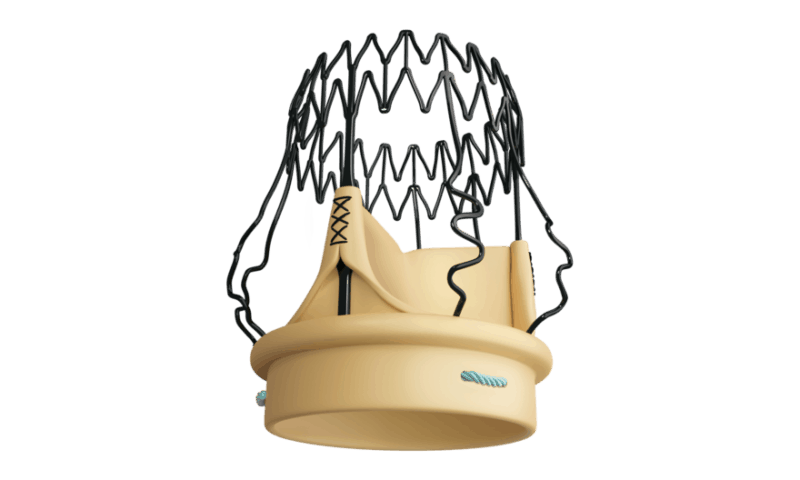
LivaNova delivered new data from its sutureless aortic heart valve, showing fewer complications and stronger clinical outcomes compared to traditional implants built on a stent frame.
An international study enrolled 578 patients who underwent a procedure to replace a severely narrowing aortic valve, which releases blood out of the heart’s left ventricle on its way to the rest of the body. They were randomized to receive either the company’s Perceval implant or another stented, biological valve.
Those who received the Perceval implant, placed through a small incision in the upper portion of the breastbone, had significantly lower rates of major cardiovascular and cerebrovascular events, including strokes, aneurysms, heart attacks and death. After one year, a composite measurement reached 5.2% in the Perceval group, compared to 10.8% among those who received stented valves.
LivaNova’s valve also saw fewer cases of new atrial fibrillation, at 4.2% versus 11.4%, as well as fewer subsequent strokes, at 1% compared to 5.4%, resulting in a lower overall number of rehospitalizations, the company said.
In addition, during the procedure to place the sutureless implant, surgeons reported a 30% reduction in the amount of time needed to clamp down the aorta and restrict the heart’s blood flow while securing the valve. The study’s results were presented during the annual meeting of the European Association for Cardio-Thoracic Surgery.
Meanwhile, a separate international registry using real-world data to compare patient outcomes found that the placement of the Perceval valve through minimally invasive procedures showed similar success rates compared to a full incision through the sternum. The minimally invasive route also resulted in shorter hospital stays and less time in intensive care.
The registry also tracked LivaNova’s newer Perceval Plus implant design, showing that fewer treated patients would go on to require a pacemaker implant compared to the original Perceval valve.
The Perceval valve has been implanted in more than 50,000 patients since it received a CE Mark in 2011 and FDA approval in 2016, the company said, while its Perceval Plus secured a CE Mark in 2018.