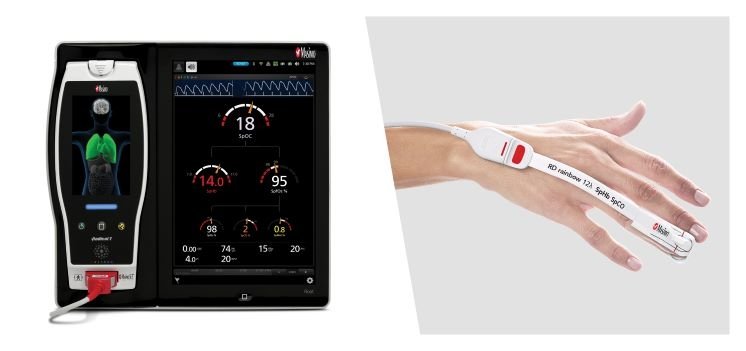
Normally, chasing rainbows is an inherently futile act. Masimo’s pursuit, however, is the rare one proven fruitful, with a new CE mark for its Rainbow SuperSensor.
The single-use, adhesive SuperSensor is attached to a patient’s fingertip, where it collects continuous readings of a dozen health parameters and sends them to a connected monitor from either Masimo or another devicemaker.
The collected data points include functional and fractional oxygen saturation, oxygen reserve index, oxygen content, pulse rate, respiration rate, perfusion index, single- and multi-wavelength measurements of pleth variability index and hemoglobin, carboxyhemoglobin and methemoglobin.
Each of the 12 blood parameters is monitored noninvasively by one of 12 LED sensors built into the device, all of which can collect readings simultaneously, allowing healthcare providers to constantly monitor the status of high-risk patients.
The Rainbow SuperSensor’s latest CE mark and subsequent rollout follows a 2017 European green light for an earlier version of the device. At that time, the Rainbow SuperSensor was designed as a reusable spot-check sensor—rather than for continuous monitoring of a single patient—and was equipped with LED sensors to collect data on only about half of the blood parameters of the latest iteration.
The new additions are the sensors to monitor fractional oxygen saturation, oxygen reserve index, oxygen content, respiration rate and multi-wavelength readings of PVi.
Adding the sensor for oxygen reserve index, for one, could potentially give clinical teams an earlier warning of hypoxemia or hyperoxia in patients receiving supplemental oxygen before it occurs, since it offers a more precise measurement of oxygenation than standard pulse oximetry, according to Masimo.
With the fractional oxygen saturation sensor, meanwhile, clinicians can better identify potential impairments to blood oxygenation in patients with elevated levels of dyshemoglobin—a form of hemoglobin incapable of being oxygenated—rather than relying solely on functional saturation readings, which only take into account the levels of hemoglobin capable of being oxygenated.
The Rainbow SuperSensor is not yet available in the U.S., where Masimo’s oxygen reserve index, multi-wavelength PVi and fractional oxygen saturation sensors have yet to receive FDA clearance.
Still, Masimo has racked up plenty of other clearances for its patient monitoring technologies both in the U.S. and abroad. Earlier this year, for example, the FDA bestowed a 510(k) clearance on the company’s Radius PCG wireless, hand-held device, a capnograph used for continuous measurement of end-tidal carbon dioxide and respiration rate.
And in June, Masimo received another FDA green light, this one for its Radius T°. Another wireless, Bluetooth-connected device, the Radius T° is a wearable thermometer that sends continuous temperature readings to a connected patient monitoring platform, cleared for both prescription and over-the-counter use in patients ages five and older.