Could native, exhausted and no-longer-functioning natural killer cells (NK cells) in cancer patients be rescued and revived by a novel, off-the-shelf immuno-oncology therapeutic that also happens to be a pervasive serial cancer killer?
The Data Says Yes.
Initial human data of GT Biopharma’s disruptive, first-in-class TriKE™ therapy shows that a patient’s native Natural Killer (NK) cells are rescued from their state of exhaustion and inability to kill cancer cells without the need for administration of supplemental ex vivo (outside the body) engineered donor or autologous NK cells, or NK cells induced from progenitors (e.g. induced pluripotent stem cells or cord blood), which is a costly manufacturing process added to an already costly therapeutic regiment.
Unlike cell therapies, TriKE™ exerts its therapeutic effect in patients without the need for the administration of engineered progenitor-derived or allogenic/autologous cells. TriKE™ is a protein biologic immuno-oncology therapeutic incorporating an NK cell activating domain, Interleukin-15 (IL-15) to aid NK cell proliferation and persistence, and a cancer cell targeting domain. TriKE™ therapy is well tolerated by patients, and does not provoke cytokine release syndrome (CRS) which is common with bispecific T-cell engager (BiTE®) or CAR-T cell therapies. Cytokine release syndrome is caused by a large, rapid release of cytokines into the blood from hyperactivated immune T cells which can cause organ failure and be life threatening.
GTB-3550 is the Company’s lead TriKE™ therapeutic candidate now being evaluated in patients in a clinical trial for the treatment of relapsed/refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) (clinicaltrials.gov – NCT03214666). AML and MDS are cancers of the cells in the bone marrow which differentiate into blood cells. TriKE™ is able to activate and promote serial killing of cancer cells by the patient’s NK cells. Thus far, patients treated with TriKE™ have shown no significant side effects.
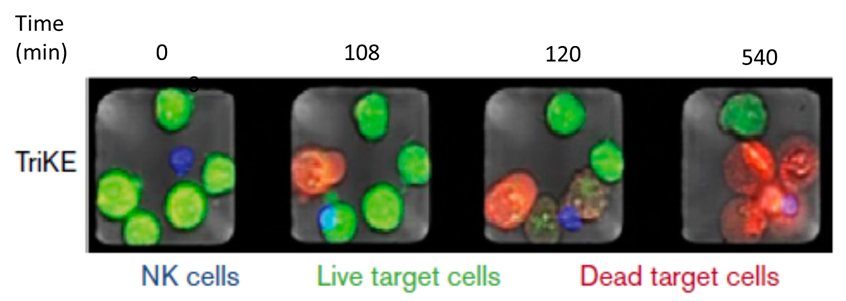
Miller, J.S., et al., Blood Advances, 16 June 2018 – Volume 2, Number 12
GT Biopharma (NASDAQ: GTBP) has an exclusive worldwide license with the University of Minnesota to further develop and commercialize therapies using TriKE™ technology. Dr. Jeffrey S. Miller, Professor of Medicine and GT Biopharma’s Consulting Chief Medical Officer, and his colleagues developed TriKE™.
“We have shown that GTB-3550 can mediate CD16 directed cytotoxicity against CD33 cancer targets. The IL-15 activity is fully preserved in patients, and allows us to redirect NK cells to malignant targets while providing cytokine stimulation with an easily exportable, off-the-shelf, non-cell therapy strategy which promotes activation, proliferation and persistence of the patient’s own endogenous NK cells,” stated Dr. Miller.
Data presented at the recent American Society of Hematology (ASH) meeting last December, in the open-label dose escalation part of the GTB-3550 Phase I/II expansion clinical trial, demonstrated immediately prior to commencing TriKE™ therapy a patient’s NK cells have no ability to kill cancer cells. GTB-3550 TriKE™ was able to activate and target-direct the patients’ NK cells to kill the AML and MDS blast cancer cells leading to a significant reduction in cancer burden and clinical benefit. This clinical achievement allowed the patient to become eligible for and obtain a bone marrow/HSC transplant, which is the only therapy generally considered to be a potentially curative therapy for AML and MDS patients.
Unlike progenitor derived NK cell therapies and other expensive autologous/allogenic NK cell therapies, GTB-3550 TriKE™ clinical data demonstrates a protein biologic can safely activate and harness the patient’s native NK cells’ cancer killing ability in a target-directed manner. TriKE™ therapies are off-the-shelf protein biologics administered as a monotherapy by infusion. TriKE™ therapies are designed to rescue the patient’s native NK cell population, and be a self-sustaining, self-activating, target-directed NK cell therapeutic agent without the need to transplant NK cells in patients or be co-administered with other therapeutics.
TriKE™ is a trademark of GT Biopharma, Inc.
BiTE® is a registered trademark of Amgen, Inc.
GT Biopharma, Inc. (NASDAQ: GTBP) is a clinical stage biopharmaceutical company focused on the development and commercialization of immuno-oncology therapeutic products based on our proprietary TriKE™ NK cell engager platform. The TriKE™ platform is designed to activate and redirect the target cell killing abilities of the patient’s natural killer cells (NK cells) without the need for supplemental ex vivo engineered donor or autologous NK cells, or induced pluripotent stem cells (iPSC). The Company has an exclusive worldwide license agreement with the University of Minnesota to further develop and commercialize therapies using TriKE™ technology.