Study results that Novocure presented at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago on Tuesday scratched a seven-year itch: According to the company, its study is the first phase 3 clinical trial in at least as long to detail a therapy’s ability to significantly improve the survival rate for patients with platinum-resistant, metastatic non-small cell lung cancer (NSCLC).
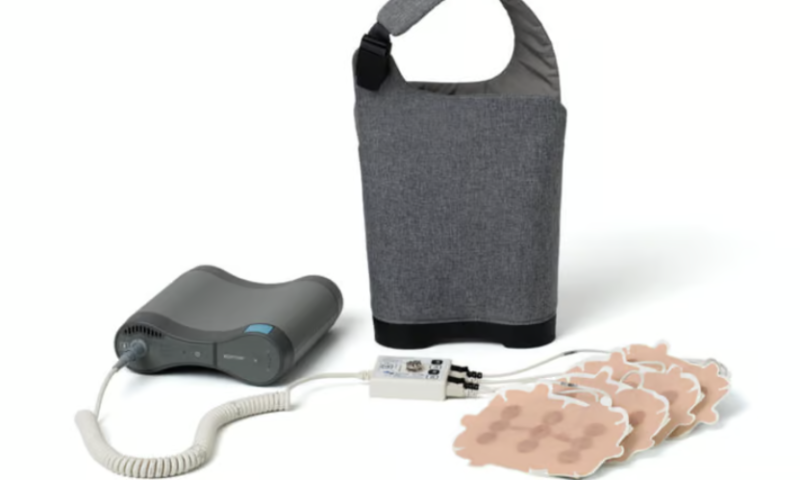
The study revolved around Novocure’s Tumor Treating Fields technology, or TTFields, which generates relatively high-frequency electric fields with an aim of interrupting cancer cells’ division and reproduction.
The technology, which was approved by the FDA in 2011 to help treat glioblastoma, has been under development for more than two decades, William Doyle, Novocure’s executive chairman, said in an interview with Fierce Medtech at ASCO.
“What’s exciting is 23 years later, we’ve now moved beyond the brain and shown the first positive phase 3 results in the rest of the body,” he said. “We now have proven that it’s not just for the localized, special case of the brain, and not only are we going to be able to help all these patients with lung cancer, but it now is the proof of principle that we can treat all of the nasty solid tumors of the trunk.”
For Novocure’s latest study, dubbed the LUNAR trial, researchers recruited a total of 276 people around the world with advanced NSCLC for whom platinum-based therapies had failed. They were randomized to receive a current standard-of-care treatment—either an immune checkpoint inhibitor or the chemotherapy medication docetaxel—or a combination of one of the drugs and TTFields.
After tracking each participant’s progress for at least a year, the researchers calculated a median overall survival rate of 10 months for those treated only with standard therapies, but just over 13 months for those who had TTFields added to the mix.
The technology’s potential benefits stretched even further when the study’s authors zoomed in only on the participants whose standard-of-care therapy had been of the immune checkpoint inhibitor variety—which is now the first-line treatment for about 70% of advanced NSCLC patients in the U.S., Doyle noted in the interview. Those who received the drug alone demonstrated a median survival rate of 10.8 months, versus 18.5 months for those receiving an ICI and TTFields.
“We take this super hard-to-treat patient population, and we’re delivering really clinically meaningful improvement in overall survival in a disease where lots of other things have been tried and nothing has moved the needle. But when we combine it with immune checkpoint inhibitors, we really move the needle,” Doyle said.
The docetaxel group, meanwhile, experienced overall survival of 8.7 months without TTFields and 11.1 months with the electrical approach—a subgroup that “didn’t hit statistical significance, but showed the positive trend,” according to the chairman.
However, despite those added months of overall survival across the TTFields group, changes in their median progression-free survival were limited. According to Novocure, the standard-of-care group went 4.1 months before the disease began to worsen again, while those who also received TTFields therapy experienced just a few extra weeks without any progression, for a total of 4.8 months.
Additionally, almost all of the TTFields recipients experienced adverse events during the treatment period, though Novocure noted that only 71% were directly related to the technology, and the majority of those were grade 1 and 2 skin irritation—with no severe (grade 4 or 5) adverse events. The technology wasn’t responsible for any patient deaths, nor did it add any systemic toxicities for users.
Next up, not only is Novocure planning to submit the LUNAR study results to the FDA as part of a premarket approval application for TTFields’ use in NSCLC, but it will also look into introducing TTFields earlier in the treatment process for metastatic NSCLC, rather than as only a second-line therapy after gauging a patient’s response to platinum-based drugs.
“This is where I get kicked under the table by my IR people, but this should absolutely go to the first line,” Doyle said. “With a therapy that shows this great synergy without toxicity, that’s where we’ll go next.”
Meanwhile, Novocure is also in the midst of studying the technology’s use in three other indications. Results of those phase 3 trials—in ovarian cancer, brain metastases and pancreatic cancer, chronologically—are expected to be published by the end of 2024.
The TTFields tech is being tested alongside different combinations of drugs in each study, Doyle said, but has typically produced the greatest results to date when administered with an immune checkpoint inhibitor—even, in some cases, with immunotherapy drugs that haven’t been particularly successful on their own.
“Every trial is different, but what we have shown consistently is that if we can deliver these electric fields at the therapeutic intensity—which we know: It’s one to three volts per centimeter—and if the patient uses it, then we know we can affect the disease,” he said. “We’re learning which combinations are the best, but we always see a benefit.”