Pfizer inadvertently gave a leg up to two obesity biotechs yesterday with the announcement that an extended-release formulation of oral med danuglipron will be advanced into dose optimization studies.
The New York-based Big Pharma is hoping to recover from earlier data that showed a twice-daily formulation of oral danuglipron led to modest weight loss with a high level of adverse events and patient discontinuations.
Now, the company is looking to advance the med as a once-daily, modified-release formulation on the strength of “encouraging pharmacokinetic data.”
Analysts at Mizuho saw the event as validation of Terns Pharmaceuticals’ oral drug, while Raymond James analysts called out Pfizer for its dosing strategy and saw a possible “tailwind” for Viking Therapeutics.
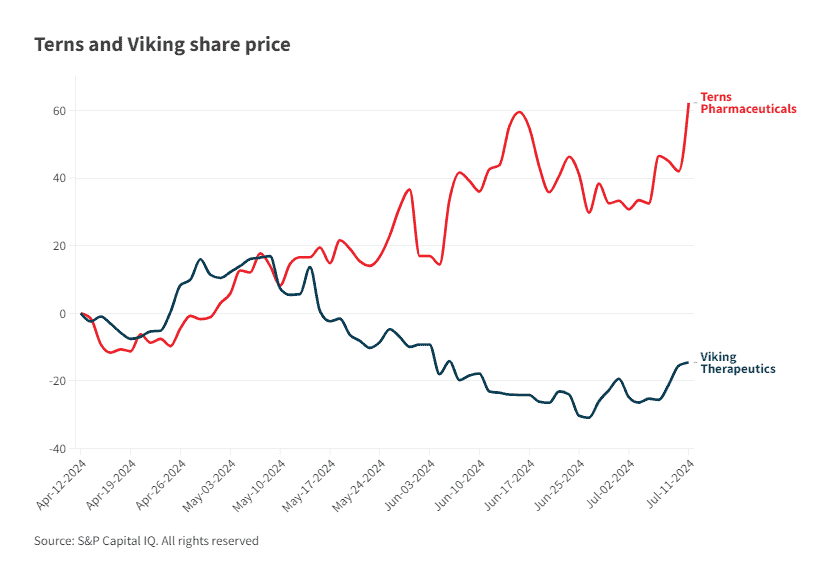
Both biotechs saw their shares rise following Pfizer’s disclosure on Thursday, although Terns was the clear winner. The company’s shares reached a peak of $9.20 Thursday from a Wednesday closing price of $7.61. Heading into Friday, Terns was trading up 4% at $8.70.
Viking’s shares had a more subtle—and temporary—bump Thursday, after closing Wednesday at about $58 apiece. The company hit a high of $60 Thursday before settling at just over $58 again to close the day.
But Viking has managed to help its own fortunes plenty. In February, the company’s shares jumped to a high of $94 when it shared the news that the dual agonist of GLP-1 and GIP VK2735 achieved weight loss of 9.1% to 14.7% in a phase 2 trial.
Raymond James’ team did not see the Pfizer news as negative for Viking, instead suggesting that the dosing strategy could eventually end with little to show.
“All told, Pfizer advancing this molecule into a dose finding study has zero negative readthrough to VKTX in our view, and may in fact be a tailwind if or when the dose-finding data are reported and look uncompetitive like the [twice a day] data,” Raymond James wrote in a Thursday note.
Linking to Pfizer’s own danuglipron press release from December 2023, Raymond James continued: “The annals of biotech are of course littered with examples of drugs that failed as [twice a day] formulations (see here), and pivoted to a [once a day] formulation with great success in hyper competitive market segments (/end sarcasm).”
In Pfizer’s own study, patients who took any less than 80 mg danuglipron twice a day had essentially no difference in weight loss compared to placebo, Raymond James pointed out. An earlier examination of 200 mg once a day also showed less exposure than a twice-a-day formulation.
Similar to Raymond James, Leerink Partners felt the Pfizer disclosure left more to be desired. The pharma giant still has a lot of work to do before danuglipron is truly validated.
“Our view is that it remains to be seen whether [once daily] danu offers competitive efficacy, tolerability, safety, and administration, and we would not expect efficacy and tolerability results in obese patients until 2026 at the earliest,” Leerink Partners wrote on Thursday.
The firm also pointed to the lack of potency seen in earlier studies at a once-daily dose.
Over at Terns, the news brought a more obvious reward for investors. Mizuho’s analysts noted that the biotech’s GLP-1 RA therapy TERN-601 was originally modeled on danuglipron, so the news is a positive for the company. Terns is also examining a once-daily dose, so the Pfizer advancement “provides us an element of increased comfort and reassurance around ‘601’s prospects,” Mizuho wrote in a Thursday note.
Top-line data for the candidate are expected in the second half of the year, or any day now, Mizuho noted.

