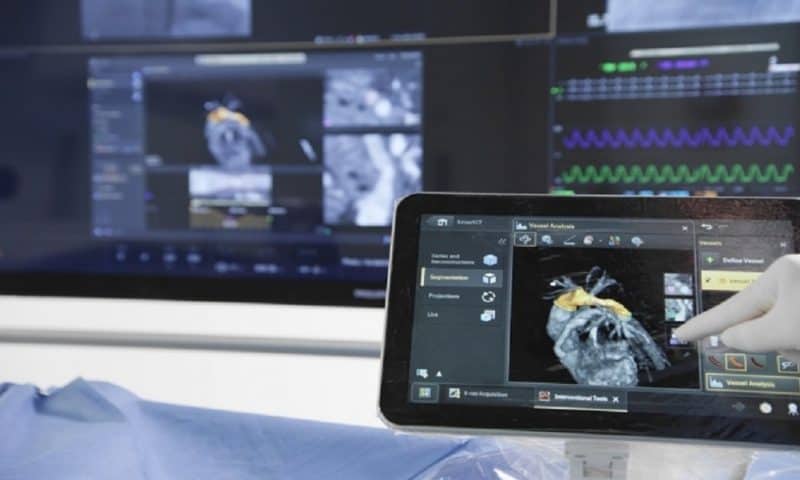
The FDA didn’t need 3D glasses to see the potential benefits of Philips’ new three-dimensional imaging software, as evidenced by the 510(k) clearance the agency recently granted to its SmartCT program.
Philips unveiled the software in September 2020 alongside the relaunch of its Azurion image-guided therapy platform. Within the Azurion system, SmartCT guides clinicians through the acquisition and analysis of 3D imaging data collected via angiography, neurology, soft-tissue imaging and guidewire navigation.
Clinicians manipulate and measure the CT-like scans on a touchscreen tablet. Uses for the 3D technology include isolating specific blood vessels, tracking an inserted catheter from multiple angles and erasing structures blocking the area of focus, and capturing and storing images throughout each process.
The tablet can be brought directly into the procedure room and connected to a larger monitor there, allowing for more timely and effective diagnosis and treatment of conditions requiring interventional radiology procedures such as aneurysms, liver tumors and vascular diseases.
“A key part of our image-guided therapy strategy is to combine high-quality, low X-ray dose imaging with a superior user experience that allows interventional radiologists to diagnose and treat patients as part of smoother, safer and less interrupted workflows,” Ronald Tabaksblat, Philips’ general manager of image-guided therapy systems, said in a release.
“Philips SmartCT is a major step forward in 3D imaging, enhancing confidence in the interventional suite and supporting key elements of the quadruple aim of better patient outcomes, enhanced patient and staff experiences and lower cost of care,” Tabaksblat said.
SmartCT integrates directly into the Azurion platform, which was initially launched in 2017 and was revamped last year. The newest version combines imaging software like SmartCT with several other applications for blood flow, patient monitoring and informatics, all housed on the portable in-lab tablet.
As of Azurion’s September relaunch, the platform had been used in more than 2 million procedures around the world, Philips said at the time. With the FDA nod, Azurion users in the U.S. will now be able to purchase and add the SmartCT software to the platform, supporting Philips’ growing investment in and reliance on its image-guided therapies business.
According to the company’s year-end report, in 2020, image-guided therapy made up almost one-third of all sales in Philips’ diagnosis and treatment segment, topped only by the closely related diagnostic imaging business. In turn, the diagnosis and treatment segment accounted for more than 40% of the company’s total sales in spite of COVID-induced slowdowns.
Looking ahead, CEO Frans van Houten wrote in the report, Philips is banking on “solid growth” in the diagnosis and treatment sector to boost sales in 2021, no doubt driven in large part by sales of the Azurion platform and accompanying software like SmartCT.