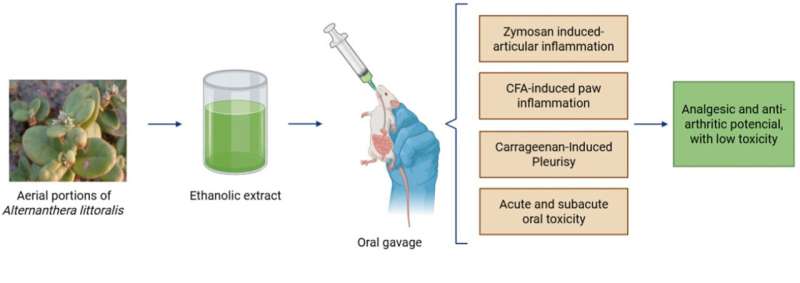
In Brazil, researchers from the Federal University of Grande Dourados (UFGD), the State University of Campinas (UNICAMP), and São Paulo State University (UNESP) have conducted a study that confirmed the safety and anti-inflammatory, analgesic, and anti-arthritic properties of the Joseph’s Coat plant (Alternanthera littoralis).
Native to the Brazilian coast, this plant has been used in folk medicine to combat inflammation, microbial infections, and parasitic diseases. Until now, there has been little pharmacological evidence to support these applications or analyze their safety.
The study is published in the Journal of Ethnopharmacology.
The first step of the study was to conduct phytochemical analyses of the plant to identify the main bioactive compounds in the ethanolic extract of its aerial parts. This analysis was conducted by Marcos Salvador, a pharmacist from the Institute of Biology (IB) at UNICAMP. Next, the team led by pharmacologist Cândida Kassuya from the Faculty of Health Sciences at UFGD evaluated the anti-inflammatory efficacy in experimental models of arthritis.
“Finally, we performed the toxicological analyses under my coordination,” explains Arielle Cristina Arena, associate professor in the Department of Structural and Functional Biology at the Institute of Biosciences at UNESP’s Botucatu Campus.
The results showed that the ethanolic extract of A. littoralis significantly reduces inflammation in laboratory animals.
“In the experimental models, we observed reduced edema, improved joint parameters, and modulation of inflammatory mediators, suggesting antioxidant and tissue-protective actions,” says Arena.
According to the professor, the findings reinforce the plant’s medicinal potential and establish a solid scientific basis for future preclinical research and the possible development of herbal products. The conclusions suggest a safety profile at therapeutic doses that may also be promising for human use.
Despite the encouraging outcome, it is not yet possible to recommend its immediate clinical use. Further toxicological analyses, as well as clinical studies and the standardization of the extract, are needed to ensure safety, efficacy, and pharmacotechnical quality. Additionally, the path to therapeutic application requires further regulatory steps.
“This research is part of an ongoing line of investigation developed by UFGD, UNESP, and UNICAMP, and our purpose is to value Brazilian biodiversity and traditional knowledge, but with a rigorous scientific basis, promoting the safe and rational use of natural products,” says Arena.