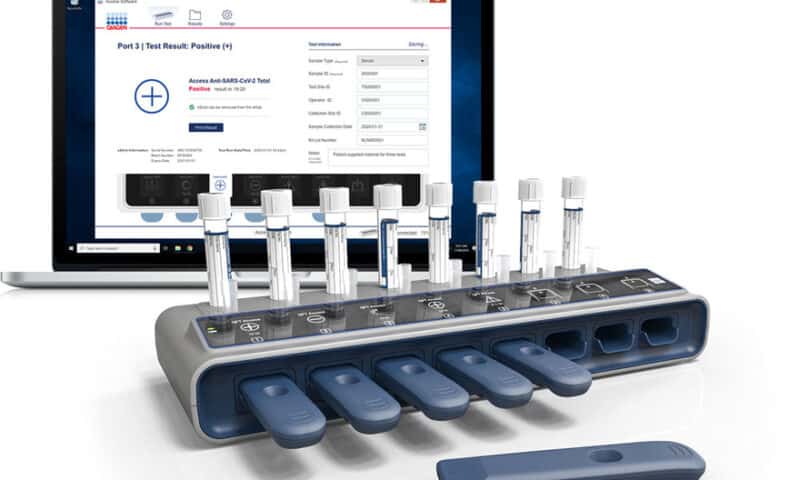
Qiagen has announced plans to launch a new digital test for COVID-19 antibodies that can run multiple samples at once on a small portable device and deliver results in about 10 minutes.
With a small digital hub and interchangeable testing stick system, the eHub was developed for use against the novel coronavirus in collaboration with Australian diagnostic developer Ellume. The device can process up to eight samples simultaneously, or up to 32 tests per hour, to detect the presence of IgA, IgM or IgG antibodies in plasma or serum.
The same platform has been used for Qiagen’s QuantiFERON-TB Access test for latent tuberculosis infections in low-resource regions. Compared to single-strip, lateral-flow antibody tests, the device can automate and standardize the readout of results, freeing up lab staff who no longer have to manually inspect and record the outcome of each sample.
“Increased testing is the only way to gain visibility on the magnitude of the pandemic, which will ultimately lead to helping control it,” Qiagen Chief Medical Officer Davide Manissero said. “This is a rugged and portable platform that requires no hardware, can process a wide range of tests and provides fast results.”
According to Qiagen and Ellume, the test has shown it can perform with zero false-positive or false-negative readings from samples taken two weeks after a person first displayed symptoms.
The test has been submitted to the FDA for emergency review, but Qiagen aims to begin rolling out the diagnostic before the end of this month. The company has preordered 900,000 tests, with the first batch already shipped to the U.S., according to Ellume.
Currently, agency regulations allow coronavirus test developers to begin shipping their product before receiving an official authorization, as long as the FDA is notified and the test has passed internal validation studies. Qiagen and Ellume also plan to secure a CE mark in Europe within the coming weeks.