As competitor Philips continues to grapple with the effects of its long-standing recall of continuous positive airway pressure (CPAP) machines and other respiratory devices, ResMed is now issuing a safety warning of its own.
The warning was billed as an urgent field safety notice rather than a formal recall. Patients were sent a letter (PDF) this week detailing an update to the “Contraindications and Warnings” sections of the user guides for certain ResMed devices.
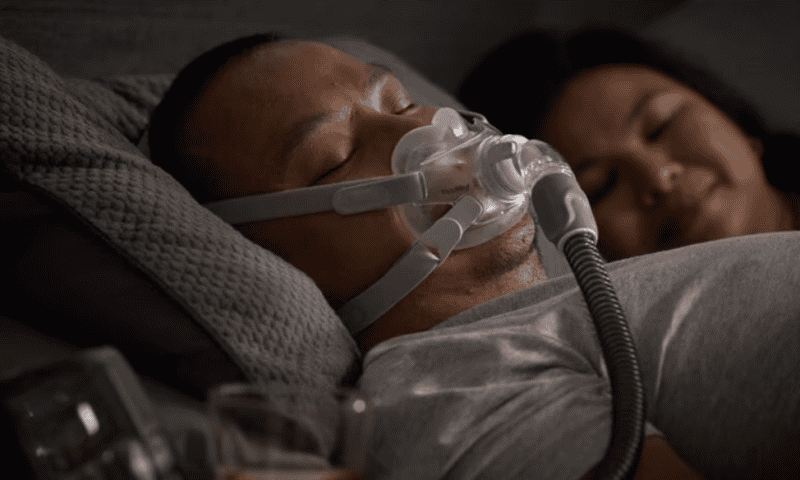
Those affected devices include a variety of full face masks, nasal masks and non-vented masks that are used to deliver PAP therapy and contain magnets. The magnets are embedded in the frame and lower headgear clips of the masks and are included to make it easier for users to make adjustments to the fit of the masks.
According to the Nov. 20 letter, ResMed has updated the user guides for the affected masks to make it clear that the embedded magnets could potentially interfere with certain implanted medical devices and metal-based implants.
The former category includes pacemakers, neurostimulators and insulin pumps, among others—the functions of which could potentially be interrupted by magnetic interference from a CPAP mask. Meanwhile, the masks’ magnets could also shift the positions of certain metal implants placed throughout the body such as stents, valves, screws, electrodes, cranial plates and more.
Either form of interference could potentially lead to serious injuries or death, though ResMed classified those as “rare circumstances.”
It noted in the letter that of the “tens of millions” of magnet-equipped masks that it’s sold around the world between 2014 and now, ResMed has only received five reports of serious harm potentially related to the magnet issue that had to be passed on to the relevant authorities. The cases included interference with implantable cardioverter defibrillators and cerebrospinal fluid shunts, and none resulted in permanent injuries or deaths, according to the company.
In the updated instructions for use, ResMed warned that magnet-equipped masks should always be kept at least six inches away from any implants that may be susceptible to magnetic interference—including both users’ own implants and those of anyone they may be near while wearing the masks. It recommended that users who have or are often physically close to anyone who has any of the contraindicated implants switch to a mask that doesn’t contain magnets to avoid the risk of interference.
Philips alerted users of its own magnetic masks to the same issue last year, though it took the formal recall route to update the masks’ instructions and labeling. In the initial September 2022 notice, the Dutch devicemaker said it had so far received 14 reports linking the CPAP and BiPAP masks’ magnets to implant malfunctions that had resulted in health conditions including arrhythmia, seizures, irregular blood pressure and cognitive issues.
Though Philips originally set the scope of the recall at about 17 million masks sold around the world, an update from the FDA the following month bumped up the total to more than 22 million masks. The agency also bestowed a Class I rating—its most serious—to the recall.
Elsewhere, strong magnets have become a fixture in consumer electronics: famously in the case of Apple’s recent iterations of iPhones and its MagSafe line of chargers, mounts and accessories. The FDA re-upped its public warnings in 2021 against having those devices in close proximity with cardiac implants; don’t keep your phone in your shirt pocket if you have a pacemaker, for example. A 2022 study, meanwhile, showed that smaller accessories such as magnetic earbud cases could still be strong enough to interfere with medical hardware.