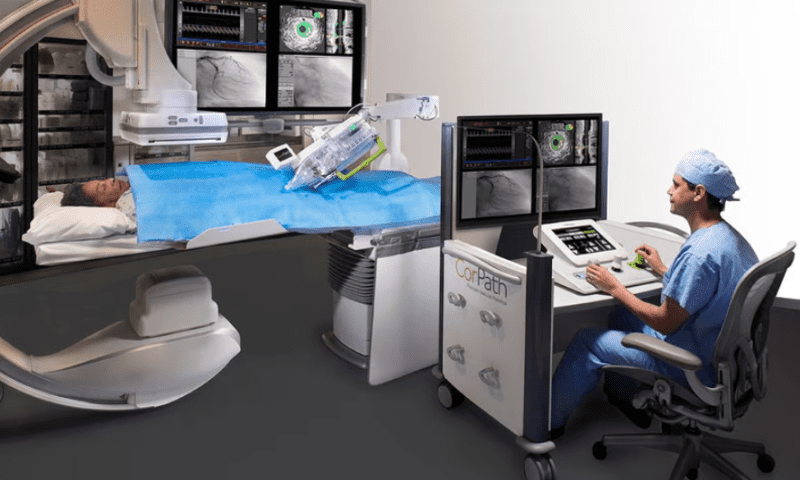
Siemens Healthineers’ Corindus division has put forward what it describes as a first-of-its-kind clinical study of the use of a surgical robot to help seal off an aneurysm within the brain.
According to the company, the CorPath GRX Neurovascular System successfully completed 94% of its robotic-assisted endovascular procedures without the surgeon having to switch to a manual approach during the operation.
The device includes a precise, remote-controlled machine that can steer a microcatheter through the blood vessels of the leg and up into the brain during live imaging procedures. After reaching the aneurysm, where the side of an artery has ballooned out and is at risk of rupturing, the device can feed coils of material into the pocket to help stem the blood flow and relieve the pressure.
More than 64% of patients saw the complete obliteration of their aneurysms, and 78% showed no clinical symptoms following the procedure. The remaining 22% were rated as a 1 or 2 on the Modified Rankin Scale for Neurologic Disability, the company said in its release, meaning they were able to continue to care for themselves without assistance and could continue most daily activities.
At the same time, the noninferiority study met its safety goals in 95.7% of cases, defined as avoiding blood vessel damage or ruptures, or any subsequent blood clotting events within the 24 hours following the procedure or the patient’s discharge from the hospital.
“Neurovascular intervention demands extreme precision to achieve optimal clinical outcomes,” the study’s principal investigator, Michel Piotin, M.D., Ph.D., head of interventional neuroradiology at the Rothschild Foundation Hospital in Paris, said in Siemens’ release. “The results of the study show the CorPath GRX System helps physicians move efficiently within tortuous and unstable vessels.”
The study results were presented at the European Society of Minimally Invasive Neurological Therapy Congress in Nice, France. It included 117 patients from 10 clinical sites in six different countries, representing a range of different aneurysm sizes, locations and characteristics.
The CorPath GRX previously received FDA and European approvals for percutaneous coronary interventions and other cardiovascular procedures. It has also received a CE mark for brain procedures; currently, Siemens is aiming to add more regulatory clearances for neurovascular indications.
“By incorporating robotic platforms in this space, we are paving the way for remote interventional procedures in the future that will connect patients to specialized interventionalists for treatment, regardless of location,” Raymond Turner, M.D., chief medical officer of neuroendovascular efforts at Corindus, said in the release.