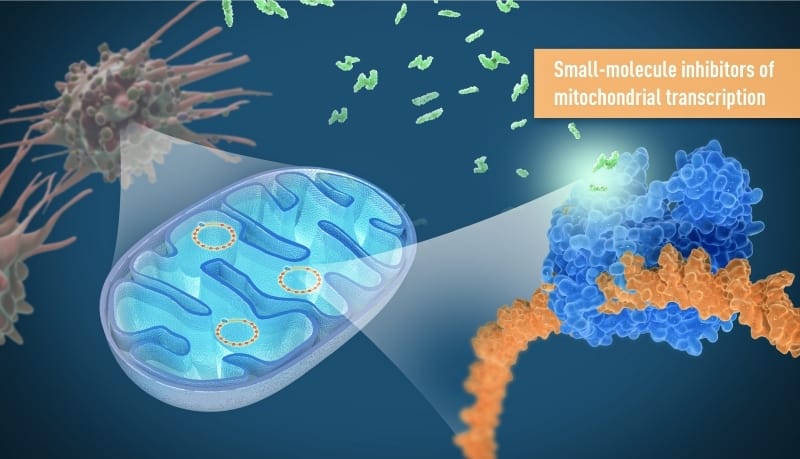
Human cells need structures called mitochondria to generate energy for their biochemical activities—and cancer cells are no exception. Researchers at the Karolinska Institutet in Sweden figured that crippling these little power plants could be a strategy for treating cancer.
Following that hypothesis, the scientists designed oral inhibitors that could target mitochondrial DNA (mtDNA). In mice, treatment with the drug led to strong anti-tumor responses, slowing tumor growth without affecting healthy cells, the team reported in Nature.
The data support further development of the drugs as potential first-in-class mitochondria inhibitors for the treatment of cancer, the researchers said in the paper.
Cancer cells need mitochondria not just for energy but also for producing several building blocks to support their wild proliferation.
Previous attempts to target mitochondria for cancer treatment often led to serious side effects because mitochondria are also key for sustaining normal cell functions.
The Karolinska-led team focused on a unique feature of cancer cells. As part of uncontrolled cell division, cancer cells must repeatedly make new mitochondria. So instead of directly targeting existing mitochondria, the team went after mitochondrial DNA transcription, which is essential to the formation of new mitochondria and the production of energy.
“Previous findings from our research group have shown that rapidly dividing cells, such as cancer cells, are crucially dependent on mtDNA to form new functional mitochondria,” Nils-Göran Larsson, the study’s senior author, explained in a statement.
The team tested a small-molecule inhibitor of mitochondrial transcription in various cancer cell lines and recorded a strong decrease in cell viability in about one-third of them. The drug wasn’t toxic to human peripheral blood mononuclear cells—which are important for immune function—or to human liver cells.
Further analysis revealed a cellular energy crisis. The treatment significantly reduced production of ATP, which carries energy in cells. It also depleted nutrients, leading to the loss of some necessary cellular building blocks.
Encouraged by the in vitro results, the researchers tested a similar drug in mouse models of ovarian and colorectal cancer. Treated animals had a clear reduction of tumor volume, the team reported. Plus, there were no signs of liver or kidney toxicity, anemia or other serious side effects after four weeks of the once-daily treatment.
Mitochondria have offered insights into cancer treatment for other research groups. A collaboration between the Mount Sinai School of Medicine and IBM found that cells with fewer mitochondria were more likely to respond to drugs that promote programmed cell death.
Research by the University of Sheffield pointed to an enzyme called TDP1, which—in releasing a protein called TOP1—helps repair mtDNA, suggesting that selectively disrupting the process might kill cancer cells.
Larsson and colleagues believe their findings suggest targeting mtDNA could be a viable cancer treatment strategy. “We are excited to have shown that this novel principle for cancer treatment works in animal models and hopefully the inhibitors can now be developed further for anti-cancer treatment in humans,” he said.