It has only recently become known that two parallel systems of blood formation exist in the body, originating from different precursor cells. Researchers at the German Cancer Research Center (DKFZ) have developed a method to examine both systems separately in mice for the first time.
Their surprising finding: The majority of immune cells do not originate from classic blood stem cells in the bone marrow, but from precursor cells that are independent of blood stem cells and are already present in the embryo. The number of these precursors decreases with age, which may explain the known decline in lymphocyte production in old age.
Blood consists of hundreds of billions of cells—from red blood cells to “white” immune cells—all of which must be constantly renewed. For decades, a simple model of blood formation was accepted: It begins with blood stem cells in the bone marrow, which differentiate into so-called multipotent progenitor cells (MPP) and finally into the various mature blood cell types.
However, in recent years, evidence has accumulated that this hierarchical model is too simplistic. New methods that allow the development of cell types to be tracked in detail have provided evidence of a second line of development of multipotent precursors—known as embryonic multipotent precursors (eMPP)—that is independent of the classic blood stem cells. These precursors arise in the early embryo and remain active into adulthood.
About one-third of blood cells originate from embryonic precursors
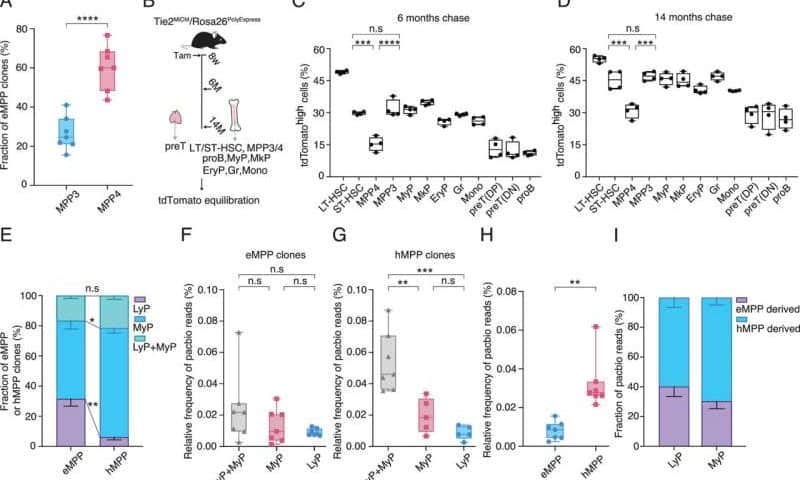
How do the two arms of blood formation differ? Do they produce different differentiated blood cells? A study of the blood-forming system in mice led by Hans-Reimer Rodewald and Thomas Höfer at the DKFZ and Xi Wang from Nanjing Medical University, China, has shed light on this discussion for the first time. The researchers developed state-of-the-art techniques for labeling individual cells in order to track the lineage and development of individual blood cell clones in mice over a period of months.
The surprising result: about one-third of blood formation originates from a system already established in the embryo (eMPP) that is independent of classic blood stem cells. This system preferentially produces lymphocytes, i.e., immune cells. The second arm, which originates from blood stem cells in the bone marrow and their descendants (hMPP), primarily produces myeloid cells such as granulocytes or erythrocytes, the red blood cells.
The researchers showed that the contribution of embryonic precursors decreases over the course of a lifetime, which may explain the decline in the effectiveness of the immune system in old age. The work is published in the Proceedings of the National Academy of Sciences.
Molecular marker for embryonic precursors discovered
In order to be able to specifically identify embryonic multipotent precursors in the future, the researchers developed a novel method they call PolySMART. This technique allows individual DNA markers, known as cellular barcodes, to be analyzed in individual cells simultaneously with surface markers and gene expression patterns.
In the process, the team discovered a surprising feature: CD138, a glycoprotein on the cell membrane, is a particularly reliable marker for eMPPs. Cells that carry CD138 are capable of self-renewal and are preferentially programmed for lymphatic development.
“With CD138, we have found the first marker that allows us to specifically isolate embryonic precursor cells and study their function,” explains Rodewald. “This opens up new avenues for understanding how the immune system changes over the course of a lifetime.”
According to the immunologist, the next step is to test whether two parallel systems are also involved in blood formation in humans. “I think this is likely, because humans and mice are similar in fundamental aspects of physiology.”
Rodewald’s colleague, bioinformatician Höfer, adds, “The fact that we have found a distinguishing feature in CD138 allows us to analyze the two blood formation systems separately for the first time. This is a milestone for stem cell research.”