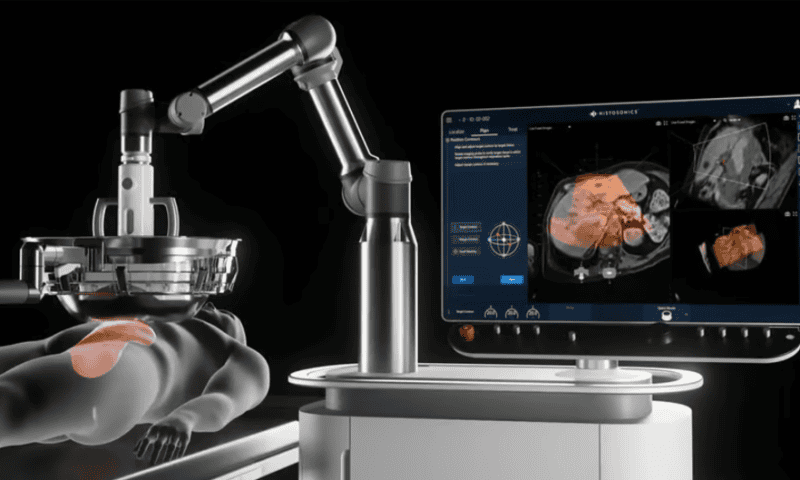
FDA allows BioNTech-MediLink ADC trial to resume with lower, safer doses
The FDA has allowed MediLink Therapeutics to restart a phase 1 trial of its BioNTech-partnered antibody-drug conjugate (ADC) that saw three fatalities after the companies confirmed that only lower doses will be used.